Regulation of in vitro human T cell development through interleukin-7 deprivation and anti-CD3 stimulation
- PMID: 22897934
- PMCID: PMC3496569
- DOI: 10.1186/1471-2172-13-46
Regulation of in vitro human T cell development through interleukin-7 deprivation and anti-CD3 stimulation
Abstract
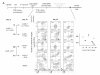
Background: The role of IL-7 and pre-TCR signaling during T cell development has been well characterized in murine but not in human system. We and others have reported that human BM hematopoietic progenitor cells (HPCs) display poor proliferation, inefficient double negative (DN) to double positive (DP) transition and no functional maturation in the in vitro OP9-Delta-like 1 (DL1) culture system.
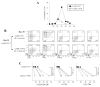
Results: In this study, we investigated the importance of optimal IL-7 and pre-TCR signaling during adult human T cell development. Using a modified OP9-DL1 culture ectopically expressing IL-7 and Fms-like tyrosine kinase 3 ligand (Flt3L), we demonstrated enhanced T cell precursor expansion. IL-7 removal at various time points during T cell development promoted a slight increase of DP cells; however, these cells did not differentiate further and underwent cell death. As pre-TCR signaling rescues DN cells from programmed cell death, we treated the culture with anti-CD3 antibody. Upon pre-TCR stimulation, the IL-7 deprived T precursors differentiated into CD3+TCRαβ+DP cells and further matured into functional CD4 T cells, albeit displayed a skewed TCR Vβ repertoire.
Conclusions: Our study establishes for the first time a critical control for differentiation and maturation of adult human T cells from HPCs by concomitant regulation of IL-7 and pre-TCR signaling.
Figures




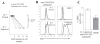


References
-
- Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, Berenson JR, Collins RH, Koup RA. Assessment of thymic output in adults after haematopoietic stemcell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–1881. doi: 10.1016/S0140-6736(00)02293-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

