Diagnostic randomized controlled trials: the final frontier
- PMID: 22897974
- PMCID: PMC3495679
- DOI: 10.1186/1745-6215-13-137
Diagnostic randomized controlled trials: the final frontier
Abstract
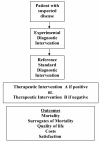
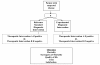
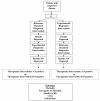
Clinicians, patients, governments, third-party payers, and the public take for granted that diagnostic tests are accurate, safe and effective. However, we may be seriously misled if we are relying on robust study design to ensure accurate, safe, and effective diagnostic tests. Properly conducted, randomized controlled trials are the gold standard for assessing the effectiveness and safety of interventions, yet are rarely conducted in the assessment of diagnostic tests. Instead, diagnostic cohort studies are commonly performed to assess the characteristics of a diagnostic test including sensitivity and specificity. While diagnostic cohort studies can inform us about the relative accuracy of an experimental diagnostic intervention compared to a reference standard, they do not inform us about whether the differences in accuracy are clinically important, or the degree of clinical importance (in other words, the impact on patient outcomes). In this commentary we provide the advantages of the diagnostic randomized controlled trial and suggest a greater awareness and uptake in their conduct. Doing so will better ensure that patients are offered diagnostic procedures that will make a clinical difference.
Figures
References
-
- Kearon C, Ginsberg JS, Douketis J, Crowther MA, Turpie AG, Bates SM, Lee A, Brill-Edwards P, Finch T, Gent M. A randomized trial of diagnostic strategies after normal proximal vein ultrasonography for suspected deep venous thrombosis: d-dimer testing compared with repeated ultrasonography. Ann Intern Med. 2005;142:490–496. - PubMed
-
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG. Standards for Reporting of Diagnostic Accuracy. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138:1W–12W. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical