LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice
- PMID: 22897998
- PMCID: PMC3501209
- DOI: 10.1016/j.jcmg.2012.06.003
LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice
Abstract
The authors investigated 3 important areas related to the clinical use of left ventricular mass (LVM): accuracy of assessments by echocardiography and cardiac magnetic resonance (CMR), the ability to predict cardiovascular outcomes, and the comparative value of different indexing methods. The recommended formula for echocardiographic estimation of LVM uses linear measurements and is based on the assumption of the left ventricle (LV) as a prolate ellipsoid of revolution. CMR permits a modeling of the LV free of cardiac geometric assumptions or acoustic window dependency, showing better accuracy and reproducibility. However, echocardiography has lower cost, easier availability, and better tolerability. From the MEDLINE database, 26 longitudinal echocardiographic studies and 5 CMR studies investigating LVM or LV hypertrophy as predictors of death or major cardiovascular outcomes were identified. LVM and LV hypertrophy were reliable cardiovascular risk predictors using both modalities. However, no study directly compared the methods for the ability to predict events, agreement in hypertrophy classification, or performance in cardiovascular risk reclassification. Indexing LVM to body surface area was the earliest normalization process used, but it seems to underestimate the prevalence of hypertrophy in obese and overweight subjects. Dividing LVM by height to the allometric power of 1.7 or 2.7 is the most promising normalization method in terms of practicality and usefulness from a clinical and scientific standpoint for scaling myocardial mass to body size. The measurement of LVM, calculation of LVM index, and classification for LV hypertrophy should be standardized by scientific societies across measurement techniques and adopted by clinicians in risk stratification and therapeutic decision making.
Copyright © 2012 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no competing interests in this study.
Figures
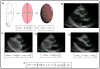



References
-
- Gidding SS. Controversies in the assessment of left ventricular mass. Hypertension. 2010;56:26–8. - PubMed
-
- Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African-American and white young adults: the CARDIA study. J Am Coll Cardiol. 2003;41:955–60. - PubMed
-
- Dannenberg AL, Levy D, Garrison RJ. Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham Study) Am J Cardiol. 1989;64:1066–8. - PubMed
-
- Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–48. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

