Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck
- PMID: 22898037
- PMCID: PMC3525135
- DOI: 10.1093/annonc/mds245
Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck
Abstract
Background: We evaluated combined targeting with cetuximab, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody, and bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, in squamous cell carcinoma of the head and neck (SCCHN).
Patients and methods: The combination was studied in human endothelial cells and head and neck and lung cancer xenograft model systems. Patients with recurrent or metastatic SCCHN were treated with weekly cetuximab and bevacizumab, 15 mg/kg on day 1 given intravenously every 21 days, until disease progression. Analysis of tumor biomarkers and related serum cytokines was performed.
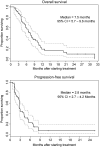
Results: Cetuximab plus bevacizumab enhanced growth inhibition both in vitro and in vivo, and resulted in potent reduction in tumor vascularization. In the clinical trial, 46 eligible patients were enrolled. The objective response rate was 16% and the disease control rate 73%. The median progression-free survival and overall survival were 2.8 and 7.5 months, respectively. Grade 3-4 adverse events were expected and occurred in less than 10% of patients. transforming growth factor alpha, placenta-derived growth factor, EGFR, VEGFR2 increased and VEGF decreased after treatment but did not correlate with treatment efficacy.
Conclusions: Cetuximab and bevacizumab are supported by preclinical observations and are well tolerated and active in previously treated patients with SCCHN.
Figures
References
-
- Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet. 2008;371:1695–1709. doi:10.1016/S0140-6736(08)60728-X. - DOI - PMC - PubMed
-
- Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222–2229. doi:10.1002/cncr.20640. - DOI - PubMed
-
- Jacobs C, Lyman G, Velez-Garcia E, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10:257–263. - PubMed
-
- Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2644–2652. doi:10.1200/JCO.2005.05.3348. - DOI - PubMed
-
- Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol. 1992;10:1245–1251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous