Study protocol for the 10 Top Tips (10TT) trial: randomised controlled trial of habit-based advice for weight control in general practice
- PMID: 22898059
- PMCID: PMC3490723
- DOI: 10.1186/1471-2458-12-667
Study protocol for the 10 Top Tips (10TT) trial: randomised controlled trial of habit-based advice for weight control in general practice
Abstract
Background: Primary care is the first port of call for advice about weight control. There is hence a need for simple, effective interventions that can be delivered without specialist skills. We have developed such an intervention; the 10 Top Tips (10TT). This intervention was effective with respect to weight loss in a volunteer population, but has yet to be tested in primary care. The aim of this trial is therefore to test the effectiveness of the 10TT intervention in primary care, incorporating clinical outcomes and health economic analyses.
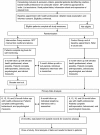
Methods/design: The trial is a two-arm, individually-randomised, controlled trial in obese (BMI ≥ 30) adults (n = 520) in primary care, comparing weight loss in patients receiving the 10TT intervention with weight loss in a control group of patients receiving usual care. The intervention is based on habit formation theory, using written materials to take people through a set of simple weight control behaviours with strategies to make them habitual; an approach that could make it more successful than others in establishing long-term behaviour change. Patients will be recruited from 14 General Practices across England. Randomisation will be through telephoning a central randomisation service using a computer-generated list of random numbers. Patients are followed up at 3, 6, 12, 18 and 24 months. The primary outcome is weight loss at 3 months, with assessment by a health professional who is blind to group allocation. Other follow-ups will be un-blinded. We will examine whether weight loss is maintained up to 24 months. We will also assess changes in the automaticity of the 10TT target behaviours and improvement in clinical markers for potential co-morbidities. Finally, we will undertake a full economic evaluation to establish cost-effectiveness in the NHS context.
Discussion: If proven to be effective when delivered through primary care, 10TT could make a highly cost-effective contribution to improvements in population health.
Trial registration: ISRCTN16347068.
Figures
References
-
- Tackling Obesities. Future Choices; www.foresight.gov.uk/Obesity/17.pdf.
-
- Counterweight Project Team. Influence of body mass index on prescribing costs and potential cost savings of a weight management programme in primary care. J Health Serv Res Policy. 2008;13(3):158–166. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous