Single electrode dynamic clamp with StdpC
- PMID: 22898473
- PMCID: PMC3482664
- DOI: 10.1016/j.jneumeth.2012.08.003
Single electrode dynamic clamp with StdpC
Abstract
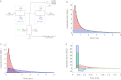
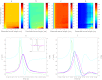
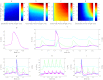
Dynamic clamp is a powerful approach for electrophysiological investigations allowing researchers to introduce artificial electrical components into target neurons to simulate ionic conductances, chemical or electrotonic inputs or connections to other cells. Due to the rapidly changing and potentially large current injections during dynamic clamp, problematic voltage artifacts appear on the electrode used to inject dynamic clamp currents into a target neuron. Dynamic clamp experiments, therefore, typically use two separate electrodes in the same cell, one for recording membrane potential and one for injecting currents. The requirement for two independent electrodes has been a limiting factor for the use of dynamic clamp in applications where dual recordings of this kind are difficult or impossible to achieve. The recent development of an active electrode compensation (AEC) method has overcome some of these prior limitations, permitting artifact-free dynamic clamp experimentation with a single electrode. Here we describe an AEC method for the free dynamic clamp software StdpC. The AEC component of StdpC is the first such system implemented for the use of non-expert users and comes with a set of semi-automated configuration and calibration procedures that facilitate its use. We briefly introduce the AEC method and its implementation in StdpC and then validate it with an electronic model cell and in two different biological preparations.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


References
-
- Alle H., Geiger J.R.P. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311(5765):1290–1293. - PubMed
-
- Brette R., Piwkowska Z., Monier C., Rudolph-Lilith M., Fournier J., Levy M., Frgnac Y., Bal T., Destexhe A. High-resolution intracellular recordings using a real-time computational model of the electrode. Neuron. 2008;59(3):379–391. - PubMed
-
- Brette R., Piwkowska Z., Rudolph M., Bal T., Destexhe A. A non-parametric electrode model for intracellular recording. Neurocomputing. 2007;70(10–12):1597–1601.
-
- Butera R., Jr., Wilson C.G., Delnegro C.A., Smith J.C. A methodology for achieving high-speed rates for artificial conductance injection in electrically excitable biological cells. IEEE Trans Biomed Eng. 2001;48(12):1460–1470. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

