Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling
- PMID: 22898544
- PMCID: PMC3519737
- DOI: 10.1186/1742-2094-9-200
Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling
Abstract
Background: Multiple adverse events are associated with the use of morphine for the treatment of chronic non-cancer pain, including opioid-induced hyperalgesia (OIH). Mechanisms of OIH are independent of opioid tolerance and may involve the morphine metabolite morphine-3-glucuronide (M3G). M3G exhibits limited affinity for opioid receptors and no analgesic effect. Previous reports suggest that M3G can act via the Toll-like receptor 4 (TLR4)/myeloid differentiation protein-2 (MD-2) heterodimer in the central nervous system to elicit pain.
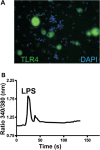
Methods: Immunoblot and immunocytochemistry methods were used to characterize the protein expression of TLR4 present in lumbar dorsal root ganglion (DRG). Using in vitro intracellular calcium and current clamp techniques, we determined whether TLR4 activation as elicited by the prototypical agonists of TLR4, lipopolysaccharide (LPS) and M3G, contributed to changes in intracellular calcium and increased excitation. Rodents were also injected with M3G to determine the degree to which M3G-induced tactile hyperalgesia could be diminished using either a small molecule inhibitor of the MD-2/TLR4 complex in rats or TLR4 knockout mice. Whole cell voltage-clamp recordings were made from small- and medium-diameter DRG neurons (25 μm < DRG diameter <45 μm) for both control and M3G-treated neurons to determine the potential influence on voltage-gated sodium channels (NaVs).
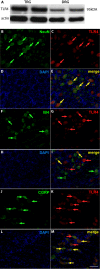
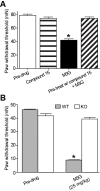
Results: We observed that TLR4 immunoreactivity was present in peptidergic and non-peptidergic sensory neurons in the DRG. Non-neuronal cells in the DRG lacked evidence of TLR4 expression. Approximately 15% of assayed small- and medium-diameter sensory neurons exhibited a change in intracellular calcium following LPS administration. Both nociceptive and non-nociceptive neurons were observed to respond, and approximately 40% of these cells were capsaicin-insensitive. Increased excitability observed in sensory neurons following LPS or M3G could be eliminated using Compound 15, a small molecule inhibitor of the TLR4/MD-2 complex. Likewise, systemic injection of M3G induced rapid tactile, but not thermal, nociceptive behavioral changes in the rat, which were prevented by pre-treating animals with Compound 15. Unlike TLR4 wild-type mice, TLR4 knockout mice did not exhibit M3G-induced hyperalgesia. As abnormal pain sensitivity is often associated with NaVs, we predicted that M3G acting via the MD-2/TLR4 complex may affect the density and gating of NaVs in sensory neurons. We show that M3G increases tetrodotoxin-sensitive and tetrodotoxin-resistant (NaV1.9) current densities.
Conclusions: These outcomes provide evidence that M3G may play a role in OIH via the TLR4/MD-2 heterodimer complex and biophysical properties of tetrodotoxin-sensitive and tetrodotoxin-resistant NaV currents.
Figures


References
-
- Zelcer N, van de Wetering K, Hillebrand M, Sarton E, Kuil A, Wielinga PR, Tephly T, Dahan A, Beijnen JH, Borst P. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc Natl Acad Sci U S A. 2005;102:7274–7279. doi: 10.1073/pnas.0502530102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

