Cryptococcus neoformans activates RhoGTPase proteins followed by protein kinase C, focal adhesion kinase, and ezrin to promote traversal across the blood-brain barrier
- PMID: 22898813
- PMCID: PMC3476282
- DOI: 10.1074/jbc.M112.389676
Cryptococcus neoformans activates RhoGTPase proteins followed by protein kinase C, focal adhesion kinase, and ezrin to promote traversal across the blood-brain barrier
Abstract
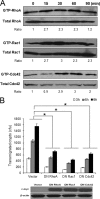
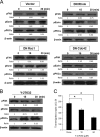
Cryptococcus neoformans is an opportunistic fungal pathogen that causes meningoencephalitis. Previous studies have demonstrated that Cryptococcus binding and invasion of human brain microvascular endothelial cells (HBMEC) is a prerequisite for transmigration across the blood-brain barrier. However, the molecular mechanism involved in the cryptococcal blood-brain barrier traversal is poorly understood. In this study we examined the signaling events in HBMEC during interaction with C. neoformans. Analysis with inhibitors revealed that cryptococcal association, invasion, and transmigration require host actin cytoskeleton rearrangement. Rho pulldown assays revealed that Cryptococcus induces activation of three members of RhoGTPases, e.g. RhoA, Rac1, and Cdc42, and their activations are required for cryptococcal transmigration across the HBMEC monolayer. Western blot analysis showed that Cryptococcus also induces phosphorylation of focal adhesion kinase (FAK), ezrin, and protein kinase C α (PKCα), all of which are involved in the rearrangement of host actin cytoskeleton. Down-regulation of FAK, ezrin, or PKCα by shRNA knockdown, dominant-negative transfection, or inhibitors significantly reduces cryptococcal ability to traverse the HBMEC monolayer, indicating their positive role in cryptococcal transmigration. In addition, activation of RhoGTPases is the upstream event for phosphorylation of FAK, ezrin, and PKCα during C. neoformans-HBMEC interaction. Taken together, our findings demonstrate that C. neoformans activates RhoGTPases and subsequently FAK, ezrin, and PKCα to promote their traversal across the HBMEC monolayer, which is the critical step for cryptococcal brain infection and development of meningitis.
Figures




References
-
- Casadevall A., Perfect J. R. (1998) Cryptococcus neoformans, American Society for Microbiology, Washington, D. C.
-
- Chayakulkeeree M., Perfect J. R. (2006) Cryptococcosis. Infect. Dis. Clin. North Am. 20, 507–544 - PubMed
-
- Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., Chiller T. M. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530 - PubMed
-
- Pukkila-Worley R., Mylonakis E. (2008) Epidemiology and management of cryptococcal meningitis. Developments and challenges. Expert Opin. Pharmacother. 9, 551–560 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

