Slow development of ALS-like spinal cord pathology in mutant valosin-containing protein gene knock-in mice
- PMID: 22898872
- PMCID: PMC3434652
- DOI: 10.1038/cddis.2012.115
Slow development of ALS-like spinal cord pathology in mutant valosin-containing protein gene knock-in mice
Abstract
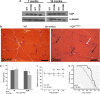
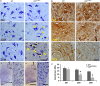
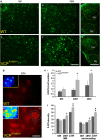
Pathological features of amyotrophic lateral sclerosis (ALS) include, in addition to selective motor neuron (MN) degeneration, the occurrence of protein aggregates, mitochondrial dysfunction and astrogliosis. SOD1 mutations cause rare familial forms of ALS and have provided the most widely studied animal models. Relatively recent studies implicating another protein, TDP-43, in familial and sporadic forms of ALS have led to the development of new animal models. More recently, mutations in the valosin-containing protein (VCP) gene linked to the human genetic disease, Inclusion Body Myopathy associated with Paget's disease of bone and frontotemporal dementia (IBMPFD), were found also to be associated with ALS in some patients. A heterozygous knock-in VCP mouse model of IBMPFD (VCP(R155H/+)) exhibited muscle, bone and brain pathology characteristic of the human disease. We have undertaken studies of spinal cord pathology in VCP(R155H/+) mice and find age-dependent degeneration of ventral horn MNs, TDP-43-positive cytosolic inclusions, mitochondrial aggregation and progressive astrogliosis. Aged animals (~24-27 months) show electromyography evidence of denervation consistent with the observed MN loss. Although these animals do not develop rapidly progressive fatal ALS-like disease during their lifespans, they recapitulate key pathological features of both human disease and other animal models of ALS, and may provide a valuable new model for studying events preceding onset of catastrophic disease.
Figures



References
-
- Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65 (Suppl 1:S3–S9. - PubMed
-
- Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5:77–87. - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

