Treatment of breast cancer stem cells with oncolytic herpes simplex virus
- PMID: 22898897
- PMCID: PMC3444761
- DOI: 10.1038/cgt.2012.49
Treatment of breast cancer stem cells with oncolytic herpes simplex virus
Abstract
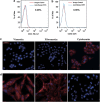
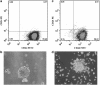
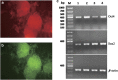
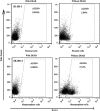
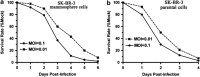
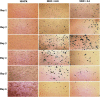
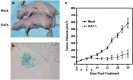
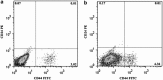
Cancer stem cells have recently been isolated from several different solid tumors. In breast cancer, the CD44(+)CD24(-/low) population is considered to comprise stem-like cells. The identification of cancer stem cells has provided new targets for the development of therapeutics. Oncolytic herpes simplex viruses (oHSVs) are an effective strategy for killing breast cancer cells and treating breast tumors in preclinical models. Here, we examined the efficacy of the oHSV G47Δ in killing breast cancer stem cells. Human breast cancer cell line SK-BR-3 and human primary breast cancer cells were cultured in suspension under conditions conducive to the growth of stem cells. They generated mammospheres, which had cancer stem cell properties. The proportion of CD44(+)CD24(-/low) cells in these mammospheres exceeded 95%, as determined by flow cytometry. The mammospheres were found to be highly tumorigenic when implanted subcutaneously in nude BALB/c mice. G47Δ contains the LacZ gene, and X-gal staining of infected cells in vitro and in vivo showed the replication and spread of the virus. G47Δ was found to be highly cytotoxic to the CD44(+)CD24(-/low) population in vitro, even when injected at low multiplicities of infection, and G47Δ treatment in vivo significantly inhibited tumor growth compared with mock treatment. This study demonstrates that oHSV is effective against breast cancer stem cells and could be a beneficial strategy for treating breast cancer patients.
Figures
Similar articles
-
Oncolytic herpes simplex virus treatment of metastatic breast cancer.Int J Oncol. 2012 Mar;40(3):757-63. doi: 10.3892/ijo.2011.1266. Epub 2011 Nov 21. Int J Oncol. 2012. PMID: 22108767
-
An oncolytic herpes simplex virus vector, G47Δ, synergizes with paclitaxel in the treatment of breast cancer.Oncol Rep. 2013 Jun;29(6):2355-61. doi: 10.3892/or.2013.2359. Epub 2013 Mar 22. Oncol Rep. 2013. PMID: 23525624
-
Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors.Cancer Res. 2009 Apr 15;69(8):3472-81. doi: 10.1158/0008-5472.CAN-08-3886. Epub 2009 Apr 7. Cancer Res. 2009. PMID: 19351838 Free PMC article.
-
Oncolytic virus therapy using genetically engineered herpes simplex viruses.Front Biosci. 2008 Jan 1;13:2060-4. doi: 10.2741/2823. Front Biosci. 2008. PMID: 17981691 Review.
-
Oncolytic Herpes Simplex Virus-Based Therapies for Cancer.Cells. 2021 Jun 18;10(6):1541. doi: 10.3390/cells10061541. Cells. 2021. PMID: 34207386 Free PMC article. Review.
Cited by
-
Triple-negative breast cancer: new perspectives for novel therapies.Med Oncol. 2013;30(3):653. doi: 10.1007/s12032-013-0653-1. Epub 2013 Jul 4. Med Oncol. 2013. PMID: 23824643 Review.
-
Oncolytic Virotherapy Treatment of Breast Cancer: Barriers and Recent Advances.Viruses. 2021 Jun 11;13(6):1128. doi: 10.3390/v13061128. Viruses. 2021. PMID: 34208264 Free PMC article. Review.
-
Treatment of orthotopic malignant peripheral nerve sheath tumors with oncolytic herpes simplex virus.Neuro Oncol. 2014 Aug;16(8):1057-66. doi: 10.1093/neuonc/not317. Epub 2014 Jan 26. Neuro Oncol. 2014. PMID: 24470552 Free PMC article.
-
VSV based virotherapy in ovarian cancer: the past, the present and …future?J Cancer. 2017 Jul 22;8(12):2369-2383. doi: 10.7150/jca.19473. eCollection 2017. J Cancer. 2017. PMID: 28819441 Free PMC article. Review.
-
Breast Cancer Stem-Like Cells in Drug Resistance: A Review of Mechanisms and Novel Therapeutic Strategies to Overcome Drug Resistance.Front Oncol. 2022 Mar 21;12:856974. doi: 10.3389/fonc.2022.856974. eCollection 2022. Front Oncol. 2022. PMID: 35392236 Free PMC article. Review.
References
-
- Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59:27–41. - PubMed
-
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. - PubMed
-
- Lawson JC, Blatch GL, Edkins AL. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res Treat. 2009;118:241–254. - PubMed
-
- Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

