Ethanol facilitates hepatitis C virus replication via up-regulation of GW182 and heat shock protein 90 in human hepatoma cells
- PMID: 22898980
- PMCID: PMC3540130
- DOI: 10.1002/hep.26010
Ethanol facilitates hepatitis C virus replication via up-regulation of GW182 and heat shock protein 90 in human hepatoma cells
Abstract
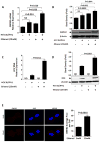
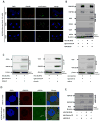
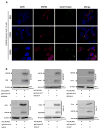
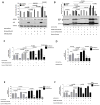
Alcohol use and hepatitis C virus (HCV) infection synergize to cause liver damage, and microRNA-122 (miR-122) appears to play a key role in this process. Argonaute 2 (Ago2), a key component of the RNA-induced silencing complex (RISC), has been shown to be important in modulating miR-122 function during HCV infection. However, GW182, a critical component of processing bodies (GW bodies) that is recruited by Ago2 to target messenger RNA (mRNA), has not been assessed in HCV infection. To characterize the role of GW182 in the pathogenesis of HCV infection, we determined its transcription and protein expression in an HCV J6/JFH1 culture system. Transcript and protein levels of GW182 as well as HCV RNA and protein expression increased with alcohol exposure. Specific silencing of mRNA expression by small interfering RNA against GW182 significantly decreased HCV RNA and protein expression. Overexpression of GW182 significantly increased HCV RNA and protein expression in HCV J6/JFH1 infected Huh7.5 cells. Furthermore, GW182 colocalized and coimmunoprecipitated with heat shock protein 90 (HSP90), which increased upon alcohol exposure with and without HCV infection and enhanced HCV gene expression. The use of an HSP90 inhibitor or knockdown of HSP90 decreased GW182 and miR-122 expression and significantly reduced HCV replication.
Conclusion: Overall, our results suggest that GW182 protein that is linked to miR-122 biogenesis and HSP90, which has been shown to stabilize the RISC, are novel host proteins that regulate HCV infection during alcohol abuse.
Copyright © 2012 American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures

Similar articles
-
Alcohol facilitates HCV RNA replication via up-regulation of miR-122 expression and inhibition of cyclin G1 in human hepatoma cells.Alcohol Clin Exp Res. 2013 Apr;37(4):599-608. doi: 10.1111/acer.12005. Epub 2012 Nov 5. Alcohol Clin Exp Res. 2013. PMID: 23126531 Free PMC article.
-
RIP-Chip analysis supports different roles for AGO2 and GW182 proteins in recruiting and processing microRNA targets.BMC Bioinformatics. 2019 Apr 18;20(Suppl 4):120. doi: 10.1186/s12859-019-2683-y. BMC Bioinformatics. 2019. PMID: 30999843 Free PMC article.
-
Human Ago2 is required for efficient microRNA 122 regulation of hepatitis C virus RNA accumulation and translation.J Virol. 2011 Mar;85(5):2342-50. doi: 10.1128/JVI.02046-10. Epub 2010 Dec 22. J Virol. 2011. PMID: 21177824 Free PMC article.
-
Function of GW182 and GW bodies in siRNA and miRNA pathways.Adv Exp Med Biol. 2013;768:71-96. doi: 10.1007/978-1-4614-5107-5_6. Adv Exp Med Biol. 2013. PMID: 23224966 Review.
-
[Regulation of hepatitis C virus genome replication by microRNA-122].Uirusu. 2015;65(2):277-286. doi: 10.2222/jsv.65.277. Uirusu. 2015. PMID: 27760927 Review. Japanese.
Cited by
-
Shedding Lights on the Extracellular Vesicles as Functional Mediator and Therapeutic Decoy for COVID-19.Life (Basel). 2023 Mar 20;13(3):840. doi: 10.3390/life13030840. Life (Basel). 2023. PMID: 36983995 Free PMC article. Review.
-
MicroRNAs in alcoholic liver disease.Semin Liver Dis. 2015 Feb;35(1):36-42. doi: 10.1055/s-0034-1397347. Epub 2015 Jan 29. Semin Liver Dis. 2015. PMID: 25632933 Free PMC article. Review.
-
Reticulon-3 modulates the incorporation of replication competent hepatitis C virus molecules for release inside infectious exosomes.PLoS One. 2020 Sep 17;15(9):e0239153. doi: 10.1371/journal.pone.0239153. eCollection 2020. PLoS One. 2020. PMID: 32941510 Free PMC article.
-
MicroRNAs: Role in Hepatitis C Virus pathogenesis.Genes Dis. 2015 Mar 1;2(1):35-45. doi: 10.1016/j.gendis.2015.01.001. Genes Dis. 2015. PMID: 25984557 Free PMC article.
-
Alcoholic liver disease and hepatitis C virus infection.World J Gastroenterol. 2016 Jan 28;22(4):1411-20. doi: 10.3748/wjg.v22.i4.1411. World J Gastroenterol. 2016. PMID: 26819510 Free PMC article. Review.
References
-
- Szabo G, Aloman C, Polyak SJ, Weinman SA, Wands J, Zakhari S. Hepatitis C infection and alcohol use: A dangerous mix for the liver and antiviral immunity. Alcohol Clin Exp Res. 2006;30:709–719. - PubMed
-
- Zhang T, Li Y, Lai JP, Douglas SD, Metzger DS, O’Brien CP, Ho WZ. Alcohol potentiates hepatitis C virus replicon expression. Hepatology. 2003;38:57–65. - PubMed
-
- McCartney EM, Semendric L, Helbig KJ, Hinze S, Jones B, Weinman SA, Beard MR. Alcohol metabolism increases the replication of hepatitis C virus and attenuates the antiviral action of interferon. J Infect Dis. 2008;198:1766–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
