C16orf57, a gene mutated in poikiloderma with neutropenia, encodes a putative phosphodiesterase responsible for the U6 snRNA 3' end modification
- PMID: 22899009
- PMCID: PMC3435495
- DOI: 10.1101/gad.193169.112
C16orf57, a gene mutated in poikiloderma with neutropenia, encodes a putative phosphodiesterase responsible for the U6 snRNA 3' end modification
Abstract
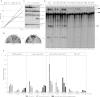
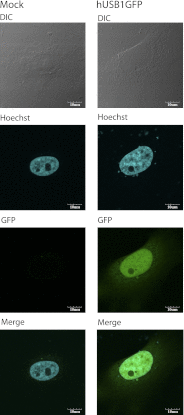
C16orf57 encodes a human protein of unknown function, and mutations in the gene occur in poikiloderma with neutropenia (PN), which is a rare, autosomal recessive disease. Interestingly, mutations in C16orf57 were also observed among patients diagnosed with Rothmund-Thomson syndrome (RTS) and dyskeratosis congenita (DC), which are caused by mutations in genes involved in DNA repair and telomere maintenance. A genetic screen in Saccharomyces cerevisiae revealed that the yeast ortholog of C16orf57, USB1 (YLR132C), is essential for U6 small nuclear RNA (snRNA) biogenesis and cell viability. Usb1 depletion destabilized U6 snRNA, leading to splicing defects and cell growth defects, which was suppressed by the presence of multiple copies of the U6 snRNA gene SNR6. Moreover, Usb1 is essential for the generation of a unique feature of U6 snRNA; namely, the 3'-terminal phosphate. RNAi experiments in human cells followed by biochemical and functional analyses confirmed that, similar to yeast, C16orf57 encodes a protein involved in the 2',3'-cyclic phosphate formation at the 3' end of U6 snRNA. Advanced bioinformatics predicted that C16orf57 encodes a phosphodiesterase whose putative catalytic activity is essential for its function in vivo. Our results predict an unexpected molecular basis for PN, DC, and RTS and provide insight into U6 snRNA 3' end formation.
Figures




Similar articles
-
Aberrant 3' oligoadenylation of spliceosomal U6 small nuclear RNA in poikiloderma with neutropenia.Blood. 2013 Feb 7;121(6):1028-38. doi: 10.1182/blood-2012-10-461491. Epub 2012 Nov 27. Blood. 2013. PMID: 23190533
-
Mutations in C16orf57 and normal-length telomeres unify a subset of patients with dyskeratosis congenita, poikiloderma with neutropenia and Rothmund-Thomson syndrome.Hum Mol Genet. 2010 Nov 15;19(22):4453-61. doi: 10.1093/hmg/ddq371. Epub 2010 Sep 3. Hum Mol Genet. 2010. PMID: 20817924 Free PMC article.
-
Structural and mechanistic basis for preferential deadenylation of U6 snRNA by Usb1.Nucleic Acids Res. 2018 Nov 30;46(21):11488-11501. doi: 10.1093/nar/gky812. Nucleic Acids Res. 2018. PMID: 30215753 Free PMC article.
-
U6 RNA biogenesis and disease association.Wiley Interdiscip Rev RNA. 2013 Sep-Oct;4(5):581-92. doi: 10.1002/wrna.1181. Epub 2013 Jun 14. Wiley Interdiscip Rev RNA. 2013. PMID: 23776162 Review.
-
The Mpn1 RNA exonuclease: cellular functions and implication in disease.FEBS Lett. 2013 Jun 27;587(13):1858-62. doi: 10.1016/j.febslet.2013.05.005. Epub 2013 May 15. FEBS Lett. 2013. PMID: 23684637 Review.
Cited by
-
Clinical utility gene card for: poikiloderma with neutropenia.Eur J Hum Genet. 2013 Oct;21(10). doi: 10.1038/ejhg.2012.298. Epub 2013 Jan 16. Eur J Hum Genet. 2013. PMID: 23321617 Free PMC article. No abstract available.
-
Rothmund-Thomson Syndrome: novel pathogenic mutations and frequencies of variants in the RECQL4 and USB1 (C16orf57) gene.Mol Genet Genomic Med. 2016 Feb 24;4(3):359-66. doi: 10.1002/mgg3.209. eCollection 2016 May. Mol Genet Genomic Med. 2016. PMID: 27247962 Free PMC article.
-
Architecture of the U6 snRNP reveals specific recognition of 3'-end processed U6 snRNA.Nat Commun. 2018 May 1;9(1):1749. doi: 10.1038/s41467-018-04145-4. Nat Commun. 2018. PMID: 29717126 Free PMC article.
-
The ribonucleotidyl transferase USIP-1 acts with SART3 to promote U6 snRNA recycling.Nucleic Acids Res. 2015 Mar 31;43(6):3344-57. doi: 10.1093/nar/gkv196. Epub 2015 Mar 9. Nucleic Acids Res. 2015. PMID: 25753661 Free PMC article.
-
The Epitranscriptome in miRNAs: Crosstalk, Detection, and Function in Cancer.Genes (Basel). 2022 Jul 21;13(7):1289. doi: 10.3390/genes13071289. Genes (Basel). 2022. PMID: 35886072 Free PMC article. Review.
References
-
- Alexander RD, Barrass JD, Dichtl B, Kos M, Obtulowicz T, Robert MC, Koper M, Karkusiewicz I, Mariconti L, Tollervey D, et al. 2010. RiboSys, a high-resolution, quantitative approach to measure the in vivo kinetics of pre-mRNA splicing and 3′-end processing in Saccharomyces cerevisiae. RNA 16: 2570–2580 - PMC - PubMed
-
- Aravind L, Koonin EV 2001. A natural classification of ribonucleases. Methods Enzymol 341: 3–28 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
