Mutations in the telomere capping complex in bone marrow failure and related syndromes
- PMID: 22899577
- PMCID: PMC3659926
- DOI: 10.3324/haematol.2012.071068
Mutations in the telomere capping complex in bone marrow failure and related syndromes
Abstract
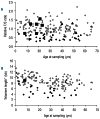
Dyskeratosis congenita and its variants have overlapping phenotypes with many disorders including Coats plus, and their underlying pathology is thought to be one of defective telomere maintenance. Recently, biallelic CTC1 mutations have been described in patients with syndromes overlapping Coats plus. CTC1, STN1 and TEN1 are part of the telomere-capping complex involved in maintaining telomeric structural integrity. Based on phenotypic overlap we screened 73 genetically uncharacterized patients with dyskeratosis congenita and related bone marrow failure syndromes for mutations in this complex. Biallelic CTC1 mutations were identified in 6 patients but none in either STN1 or TEN1. We have expanded the phenotypic spectrum associated with CTC1 mutations and report that intracranial and retinal abnormalities are not a defining feature, as well as showing that the effect of these mutations on telomere length is variable. The study also demonstrates the lack of disease-causing mutations in other components of the telomere-capping complex.
Figures

References
-
- Palm W, de Lange T. How Shelterin Protects Mammalian Telomeres. Annu Rev Genet. 2008;42:301–34 - PubMed
-
- Dewar JM, Lydall D. Similarities and differences between “uncapped” telomeres and DNA double-strand breaks. Chromosoma. 2011;121(2):117–30 - PubMed
-
- Miyake Y, Nakamura M, Nabetani A, Shimamura S, Tamura M, Yonehara S, et al. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol Cell. 2009;36(2):193–206 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

