Fine Tuning of Redox Networks on Multiheme Cytochromes from Geobacter sulfurreducens Drives Physiological Electron/Proton Energy Transduction
- PMID: 22899897
- PMCID: PMC3415244
- DOI: 10.1155/2012/298739
Fine Tuning of Redox Networks on Multiheme Cytochromes from Geobacter sulfurreducens Drives Physiological Electron/Proton Energy Transduction
Abstract
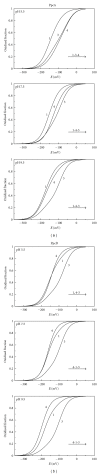
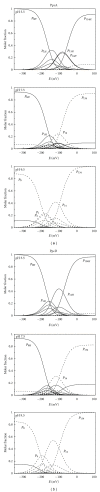
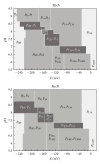
The bacterium Geobacter sulfurreducens (Gs) can grow in the presence of extracellular terminal acceptors, a property that is currently explored to harvest electricity from aquatic sediments and waste organic matter into microbial fuel cells. A family composed of five triheme cytochromes (PpcA-E) was identified in Gs. These cytochromes play a crucial role by bridging the electron transfer from oxidation of cytoplasmic donors to the cell exterior and assisting the reduction of extracellular terminal acceptors. The detailed thermodynamic characterization of such proteins showed that PpcA and PpcD have an important redox-Bohr effect that might implicate these proteins in the e(-)/H(+) coupling mechanisms to sustain cellular growth. The physiological relevance of the redox-Bohr effect in these proteins was studied by determining the fractional contribution of each individual redox-microstate at different pH values. For both proteins, oxidation progresses from a particular protonated microstate to a particular deprotonated one, over specific pH ranges. The preferred e(-)/H(+) transfer pathway established by the selected microstates indicates that both proteins are functionally designed to couple e(-)/H(+) transfer at the physiological pH range for cellular growth.
Figures
Similar articles
-
Rational engineering of Geobacter sulfurreducens electron transfer components: a foundation for building improved Geobacter-based bioelectrochemical technologies.Front Microbiol. 2015 Jul 30;6:752. doi: 10.3389/fmicb.2015.00752. eCollection 2015. Front Microbiol. 2015. PMID: 26284042 Free PMC article. Review.
-
Thermodynamic characterization of a triheme cytochrome family from Geobacter sulfurreducens reveals mechanistic and functional diversity.Biophys J. 2010 Jul 7;99(1):293-301. doi: 10.1016/j.bpj.2010.04.017. Biophys J. 2010. PMID: 20655858 Free PMC article.
-
Thermodynamic and functional characterization of the periplasmic triheme cytochrome PpcA from Geobacter metallireducens.Biochem J. 2018 Sep 14;475(17):2861-2875. doi: 10.1042/BCJ20180457. Biochem J. 2018. PMID: 30072494
-
Rational design of electron/proton transfer mechanisms in the exoelectrogenic bacteria Geobacter sulfurreducens.Biochem J. 2021 Jul 30;478(14):2871-2887. doi: 10.1042/BCJ20210365. Biochem J. 2021. PMID: 34190983
-
Diving into the redox properties of Geobacter sulfurreducens cytochromes: a model for extracellular electron transfer.Dalton Trans. 2015 May 28;44(20):9335-44. doi: 10.1039/c5dt00556f. Dalton Trans. 2015. PMID: 25906375 Review.
Cited by
-
Methane production by Methanothrix thermoacetophila via direct interspecies electron transfer with Geobacter metallireducens.mBio. 2023 Aug 31;14(4):e0036023. doi: 10.1128/mbio.00360-23. Epub 2023 Jun 12. mBio. 2023. PMID: 37306514 Free PMC article.
-
Rational engineering of Geobacter sulfurreducens electron transfer components: a foundation for building improved Geobacter-based bioelectrochemical technologies.Front Microbiol. 2015 Jul 30;6:752. doi: 10.3389/fmicb.2015.00752. eCollection 2015. Front Microbiol. 2015. PMID: 26284042 Free PMC article. Review.
-
Characterization of the inner membrane cytochrome ImcH from Geobacter reveals its importance for extracellular electron transfer and energy conservation.Protein Sci. 2023 Nov;32(11):e4796. doi: 10.1002/pro.4796. Protein Sci. 2023. PMID: 37779214 Free PMC article.
-
Biomolecular Interaction Studies Between Cytochrome PpcA From Geobacter sulfurreducens and the Electron Acceptor Ferric Nitrilotriacetate (Fe-NTA).Front Microbiol. 2018 Nov 16;9:2741. doi: 10.3389/fmicb.2018.02741. eCollection 2018. Front Microbiol. 2018. PMID: 30524391 Free PMC article.
-
Lack of Specificity in Geobacter Periplasmic Electron Transfer.J Bacteriol. 2022 Dec 20;204(12):e0032222. doi: 10.1128/jb.00322-22. Epub 2022 Nov 16. J Bacteriol. 2022. PMID: 36383007 Free PMC article.
References
-
- Lovley DR. Fe(lII) and Mn(lV) reduction. In: Press A, editor. Environmental Microbe-Metal Interactions. Washington, DC, USA: ASM Press; 2000.
-
- Lovley DR, Ueki T, Zhang T, et al. Geobacter. The microbe electric's physiology, ecology, and practical applications. Advances in Microbial Physiology. 2011;59:1–100. - PubMed
LinkOut - more resources
Full Text Sources

