O-antigen modulates infection-induced pain states
- PMID: 22899994
- PMCID: PMC3416823
- DOI: 10.1371/journal.pone.0041273
O-antigen modulates infection-induced pain states
Abstract
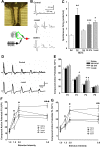
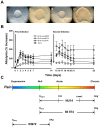
The molecular initiators of infection-associated pain are not understood. We recently found that uropathogenic E. coli (UPEC) elicited acute pelvic pain in murine urinary tract infection (UTI). UTI pain was due to E. coli lipopolysaccharide (LPS) and its receptor, TLR4, but pain was not correlated with inflammation. LPS is known to drive inflammation by interactions between the acylated lipid A component and TLR4, but the function of the O-antigen polysaccharide in host responses is unknown. Here, we examined the role of O-antigen in pain using cutaneous hypersensitivity (allodynia) to quantify pelvic pain behavior and using sacral spinal cord excitability to quantify central nervous system manifestations in murine UTI. A UPEC mutant defective for O-antigen biosynthesis induced chronic allodynia that persisted long after clearance of transient infections, but wild type UPEC evoked only acute pain. E. coli strains lacking O-antigen gene clusters had a chronic pain phenotype, and expressing cloned O-antigen gene clusters altered the pain phenotype in a predictable manner. Chronic allodynia was abrogated in TLR4-deficient mice, but inflammatory responses in wild type mice were similar among E. coli strains spanning a wide range of pain phenotypes, suggesting that O-antigen modulates pain independent of inflammation. Spinal cords of mice with chronic allodynia exhibited increased spontaneous firing and compromised short-term depression, consistent with centralized pain. Taken together, these findings suggest that O-antigen functions as a rheostat to modulate LPS-associated pain. These observations have implications for an infectious etiology of chronic pain and evolutionary modification of pathogens to alter host behaviors.
Conflict of interest statement
Figures



References
-
- Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10: 1361–1368. - PubMed
-
- Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD (2000) Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 10: 509–515. - PubMed
-
- Schaeffer AJ, Schaeffer EM (2007) Infections of the urinary tract. In: Wein A, Kavoussi L, Novick A, Partin A, Peters C, editors. Campbell-Walsh Urology. 8th ed. Philadelphia: Elsevier. pp. 223–303.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

