Analysis of uncoupling protein 2-deficient mice upon anaesthesia and sedation revealed a role for UCP2 in locomotion
- PMID: 22900002
- PMCID: PMC3416814
- DOI: 10.1371/journal.pone.0041846
Analysis of uncoupling protein 2-deficient mice upon anaesthesia and sedation revealed a role for UCP2 in locomotion
Abstract
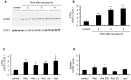
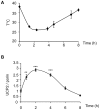
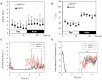
General anaesthesia is associated with hypothermia, oxidative stress, and immune depression. Uncoupling Protein (UCP2) is a member of the mitochondrial carrier family present in many organs including the spleen, the lung and the brain. A role of UCP2 in the activation of the inflammatory/immune cells, in the secretion of hormones, and in the excitability of neurons by regulating the production of reactive oxygen species has been discussed. Because of the side effects of anaesthesia listed above, we aimed to question the expression and the function of UCP2 during anaesthesia. Induction of anaesthesia with ketamine (20 mg/kg) or isoflurane (3.6%) and induction of sedation with the α2 adrenergic receptor agonist medetomidine (0.2 mg/kg) stimulated infiltration of immune cells in the lung and increased UCP2 protein content in the lung, in both immune and non-immune cells. UCP2 content in the lung inversely correlated with body temperature decrease induced by medetomidine treatment. Challenge of the Ucp2(-/-) mice with isoflurane and medetomidine revealed an earlier behavioral recovery phenotype. Transponder analysis of body temperature and activity showed no difference between Ucp2(-/-) and control mice in basal conditions. However, upon an acute decrease of body temperature induced by medetomidine, Ucp2(-/-) mice exhibited increased locomotion activity. Together, these results show that UCP2 is rapidly mobilized during anaesthesia and sedation in immune cells, and suggest a role of UCP2 in locomotion.
Conflict of interest statement
Figures


Similar articles
-
No evidence for a basal, retinoic, or superoxide-induced uncoupling activity of the uncoupling protein 2 present in spleen or lung mitochondria.J Biol Chem. 2002 Jul 19;277(29):26268-75. doi: 10.1074/jbc.M202535200. Epub 2002 May 14. J Biol Chem. 2002. PMID: 12011051
-
The uncoupling protein 2 modulates the cytokine balance in innate immunity.Cytokine. 2006 Aug;35(3-4):135-42. doi: 10.1016/j.cyto.2006.07.012. Epub 2006 Sep 12. Cytokine. 2006. PMID: 16971137
-
Mitochondrial uncoupling protein 2 protects splenocytes from oxidative stress-induced apoptosis during pathogen activation.Cell Immunol. 2013 Nov-Dec;286(1-2):39-44. doi: 10.1016/j.cellimm.2013.10.002. Epub 2013 Oct 19. Cell Immunol. 2013. PMID: 24291389
-
Uncoupling protein UCP2: when mitochondrial activity meets immunity.FEBS Lett. 2010 Apr 16;584(8):1437-42. doi: 10.1016/j.febslet.2010.03.014. Epub 2010 Mar 15. FEBS Lett. 2010. PMID: 20227410 Review.
-
Uncoupling protein-2 and the potential link between metabolism and longevity.Curr Aging Sci. 2010 Jul;3(2):102-12. doi: 10.2174/1874609811003020102. Curr Aging Sci. 2010. PMID: 20158496 Review.
Cited by
-
UCP2, a mitochondrial protein regulated at multiple levels.Cell Mol Life Sci. 2014 Apr;71(7):1171-90. doi: 10.1007/s00018-013-1407-0. Epub 2013 Jun 27. Cell Mol Life Sci. 2014. PMID: 23807210 Free PMC article. Review.
-
Comprehensive Analysis and Validation of Solute Carrier Family 25 (SLC25) and Its Correlation with Immune Infiltration in Pan-Cancer.Biomed Res Int. 2022 Oct 8;2022:4009354. doi: 10.1155/2022/4009354. eCollection 2022. Biomed Res Int. 2022. PMID: 36254139 Free PMC article.
-
Uncoupling protein 2 and 4 expression pattern during stem cell differentiation provides new insight into their putative function.PLoS One. 2014 Feb 11;9(2):e88474. doi: 10.1371/journal.pone.0088474. eCollection 2014. PLoS One. 2014. PMID: 24523901 Free PMC article.
-
lncRNA CRNDE Affects Th17/IL-17A and Inhibits Epithelial-Mesenchymal Transition in Lung Epithelial Cells Reducing Asthma Signs.Oxid Med Cell Longev. 2023 Jan 27;2023:2092184. doi: 10.1155/2023/2092184. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36743692 Free PMC article.
References
-
- Grasshoff C, Drexler B, Rudolph U, Antkowiak B (2006) Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des 12: 3665–3679. - PubMed
-
- Ohlson KB, Mohell N, Cannon B, Lindahl SG, Nedergaard J (1994) Thermogenesis in brown adipocytes is inhibited by volatile anesthetic agents. A factor contributing to hypothermia in infants? Anesthesiology 81: 176–183. - PubMed
-
- Ohlson KBE, Shabalina IG, Lennström K, Backlund EC, Mohell N, et al. (2004) Inhibitory effects of halothane on the thermogenic pathway in brown adipocytes: localization to adenylyl cyclase and mitochondrial fatty acid oxidation. Biochem Pharmacol 68: 463–477. - PubMed
-
- Alves-Guerra, MC, Pecqueur C, Shaw AZ, Couplan E, Gonzalez-Barroso, et al. (s. d.) The mitochondrial uncoupling protein family: from the cold induced thermogenesis to the regulation of ROS. Signalling and Cell Adaptation. Storey, K.B., and Storey, J.M. p. 257–268.
-
- Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, et al. (1997) Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 15: 269–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

