Stress-induced enhancement of mouse amygdalar synaptic plasticity depends on glucocorticoid and ß-adrenergic activity
- PMID: 22900007
- PMCID: PMC3416843
- DOI: 10.1371/journal.pone.0042143
Stress-induced enhancement of mouse amygdalar synaptic plasticity depends on glucocorticoid and ß-adrenergic activity
Abstract
Background: Glucocorticoid hormones, in interaction with noradrenaline, enable the consolidation of emotionally arousing and stressful experiences in rodents and humans. Such interaction is thought to occur at least partly in the basolateral nucleus of the amygdala (BLA) which is crucially involved in emotional memory formation. Extensive evidence points to long-term synaptic potentiation (LTP) as a mechanism contributing to memory formation. Here we determined in adolescent C57/Bl6 mice the effects of stress on LTP in the LA-BLA pathway and the specific roles of corticosteroid and β-adrenergic receptor activation in this process.
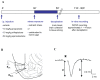
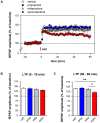
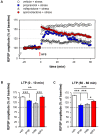
Principal findings: Exposure to 20 min of restraint stress (compared to control treatment) prior to slice preparation enhanced subsequent LTP induction in vitro, without affecting baseline fEPSP responses. The role of glucocorticoid receptors, mineralocorticoid receptors and β2-adrenoceptors in the effects of stress was studied by treating mice with the antagonists mifepristone, spironolactone or propranolol respectively (or the corresponding vehicles) prior to stress or control treatment. In undisturbed controls, mifepristone and propranolol administration in vivo did not influence LTP induced in vitro. By contrast, spironolactone caused a gradually attenuating form of LTP, both in unstressed and stressed mice. Mifepristone treatment prior to stress strongly reduced the ability to induce LTP in vitro. Propranolol normalized the stress-induced enhancement of LTP to control levels during the first 10 min after high frequency stimulation, after which synaptic responses further declined.
Conclusions: Acute stress changes BLA electrical properties such that subsequent LTP induction is facilitated. Both β-adrenergic and glucocorticoid receptors are involved in the development of these changes. Mineralocorticoid receptors are important for the maintenance of LTP in the BLA, irrespective of stress-induced changes in the circuit. The prolonged changes in BLA network function after stress may contribute to effective memory formation of emotional and stressful events.
Conflict of interest statement
Figures

Similar articles
-
Glucocorticoid receptors and beta-adrenoceptors in basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1.Neuropharmacology. 2007 Jan;52(1):244-52. doi: 10.1016/j.neuropharm.2006.07.007. Epub 2006 Aug 7. Neuropharmacology. 2007. PMID: 16890964
-
β-Adrenoceptors and synaptic plasticity in the perirhinal cortex.Neuroscience. 2014 Jul 25;273(100):163-73. doi: 10.1016/j.neuroscience.2014.04.070. Epub 2014 May 14. Neuroscience. 2014. PMID: 24836853 Free PMC article.
-
Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor--cAMP/cAMP/PKA system in influencing memory consolidation.Eur J Neurosci. 2002 Feb;15(3):553-60. doi: 10.1046/j.0953-816x.2001.01876.x. Eur J Neurosci. 2002. PMID: 11876783
-
1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation.Psychoneuroendocrinology. 2000 Apr;25(3):213-38. doi: 10.1016/s0306-4530(99)00058-x. Psychoneuroendocrinology. 2000. PMID: 10737694 Review.
-
Memory consolidation and the amygdala: a systems perspective.Trends Neurosci. 2002 Sep;25(9):456. doi: 10.1016/s0166-2236(02)02211-7. Trends Neurosci. 2002. PMID: 12183206 Review.
Cited by
-
Stress-induced anxiety-related behavior in mice is driven by enhanced excitability of ventral tegmental area GABA neurons.Front Behav Neurosci. 2024 Jul 17;18:1425607. doi: 10.3389/fnbeh.2024.1425607. eCollection 2024. Front Behav Neurosci. 2024. PMID: 39086371 Free PMC article.
-
Glucocorticoid receptor-mediated amygdalar metaplasticity underlies adaptive modulation of fear memory by stress.Elife. 2018 Jun 26;7:e34135. doi: 10.7554/eLife.34135. Elife. 2018. PMID: 29941090 Free PMC article.
-
The role of glucocorticoid receptor-dependent activity in the amygdala central nucleus and reversibility of early-life stress programmed behavior.Transl Psychiatry. 2015 Apr 7;5(4):e542. doi: 10.1038/tp.2015.35. Transl Psychiatry. 2015. PMID: 25849981 Free PMC article.
-
Enhancing memory with stress: Progress, challenges, and opportunities.Brain Cogn. 2019 Jul;133:94-105. doi: 10.1016/j.bandc.2018.11.009. Epub 2018 Dec 12. Brain Cogn. 2019. PMID: 30553573 Free PMC article. Review.
-
Role of glucocorticoid receptor-mediated mechanisms in cocaine memory enhancement.Neuropharmacology. 2017 Sep 1;123:349-358. doi: 10.1016/j.neuropharm.2017.05.022. Epub 2017 May 23. Neuropharmacology. 2017. PMID: 28549664 Free PMC article.
References
-
- Roozendaal B, McEwen BS, Chattarji S (2009) Stress, memory and the amygdala. Nat Rev Neurosci 10: 423–433. - PubMed
-
- McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28. - PubMed
-
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B (2009) Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol 30: 358–370. - PubMed
-
- Bremner JD, Krystal JH, Southwick SM, Charney DS (1996) Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 23: 28–38. - PubMed
-
- de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6: 463–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

