The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins
- PMID: 22900076
- PMCID: PMC3416761
- DOI: 10.1371/journal.pone.0042988
The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins
Abstract
Raine syndrome is caused by mutations in FAM20C, which had been reported to encode a secreted component of bone and teeth. We found that FAM20C encodes a Golgi-localized protein kinase, distantly related to the Golgi-localized kinase Four-jointed. Drosophila also encode a Golgi-localized protein kinase closely related to FAM20C. We show that FAM20C can phosphorylate secreted phosphoproteins, including both Casein and members of the SIBLING protein family, which modulate biomineralization, and we find that FAM20C phosphorylates a biologically active peptide at amino acids essential for inhibition of biomineralization. We also identify autophosphorylation of FAM20C, and characterize parameters of FAM20C's kinase activity, including its Km, pH and cation dependence, and substrate specificity. The biochemical properties of FAM20C match those of an enzymatic activity known as Golgi casein kinase. Introduction of point mutations identified in Raine syndrome patients into recombinant FAM20C impairs its normal localization and kinase activity. Our results identify FAM20C as a kinase for secreted phosphoproteins and establish a biochemical basis for Raine syndrome.
Conflict of interest statement
Figures
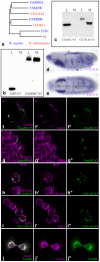
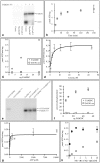

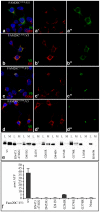
References
-
- Hulskamp G, Wieczorek D, Rieder H, Louwen F, Hornig-Franz I, et al. (2003) Raine syndrome: report of a family with three affected sibs and further delineation of the syndrome. Clin Dysmorphol 12: 153–160. - PubMed
-
- Rejjal A (1998) Raine syndrome. Am J Med Genet 78: 382–385. - PubMed
-
- Fradin M, Stoetzel C, Muller J, Koob M, Christmann D, et al. (2011) Osteosclerotic bone dysplasia in siblings with a Fam20C mutation. Clin Genet 80: 177–183. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

