Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance
- PMID: 22900129
- PMCID: PMC3412105
- DOI: 10.1155/2012/973927
Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance
Abstract
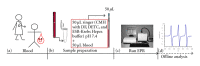
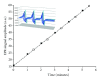
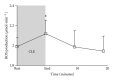
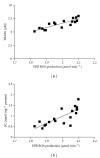
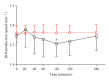
Despite the growing interest in the role of reactive oxygen species (ROS) in health and disease, reliable quantitative noninvasive methods for the assessment of oxidative stress in humans are still lacking. EPR technique, coupled to a specific spin probe (CMH: 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine) is here presented as the method of choice to gain a direct measurement of ROS in biological fluids and tissues. The study aimed at demonstrating that, differently from currently available "a posteriori" assays of ROS-induced damage by means of biomolecules (e.g., proteins and lipids) spin-trapping EPR provides direct evidence of the "instantaneous" presence of radical species in the sample and, as signal areas are proportional to the number of excited electron spins, lead to absolute concentration levels. Using a recently developed bench top continuous wave system (e-scan EPR scanner, Bruker) dealing with very low ROS concentration levels in small (50 μL) samples, we successfully monitored rapid ROS production changes in peripheral blood of athletes after controlled exercise and sedentary subjects after antioxidant supplementation. The correlation between EPR results and data obtained by various enzymatic assays (e.g., protein carbonyls and thiobarbituric acid reactive substances) was determined too. Synthetically, our method allows reliable, quick, noninvasive quantitative determination of ROS in human peripheral blood.
Figures

References
-
- Gille L, Nohl H. The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Archives of Biochemistry and Biophysics. 2001;388(1):34–38. - PubMed
-
- Holley AE, Cheeseman KH. Measuring free radical reactions in vivo . British Medical Bulletin. 1993;49(3):494–505. - PubMed
-
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicologic Pathology. 2002;30(6):620–650. - PubMed
-
- Buettner GR, Jurkiewicz BA. Ascorbate free radical as a marker of oxidative stress: an EPR study. Free Radical Biology and Medicine. 1993;14(1):49–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

