Redox reactions of myoglobin
- PMID: 22900975
- PMCID: PMC3638515
- DOI: 10.1089/ars.2012.4887
Redox reactions of myoglobin
Abstract
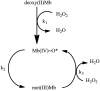
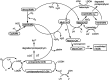
Significance: Failure to maintain myoglobin (Mb) in the reduced state causes the formation of metMb, ferryl Mb species, and cross-linked Mb. Dissociation of ferriprotoporphyrin IX from the globin and release of iron atoms can also occur as oxidized Mb accumulates. These modifications may contribute to various oxidative pathologies in muscle and muscle foods.
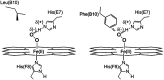
Recent advances: The mechanism of ferryl Mb-mediated oxidative damage to nearby structures has been partially elucidated. Dissociation of ferriprotoporphyrin IX from metMb occurs more readily at acidic pH values. The dissociated ferriprotoporphyrin IX (also called hemin) readily decomposes preformed lipid hydroperoxides to reactive oxygen species. Heme oxygenase as well as lipophilic free radicals can degrade the protoporphyrin IX moiety, which results in the formation of free iron.
Critical issues: The multiple pathways by which Mb can incur toxicity create difficulties in determining the major cause of oxidative damage in a particular system. Peroxides and low pH activate each of the oxidative Mb forms, ferriprotoporphyrin IX, and released iron. Determining the relative concentration of these species is technically difficult, but essential to a complete understanding of oxidative pathology in muscle tissue.
Future directions: Improved methods to assess the different pathways of Mb toxicity are needed. Although significant advances have been made in the understanding of Mb interactions with other biomolecules, further investigation is needed to understand the physical and chemical nature of these interactions.
Figures

References
-
- Alayash AI. Ryan BA. Eich RF. Olson JS. Cashon RE. Reactions of sperm whale myoglobin with hydrogen peroxide. Effects of distal pocket mutations on the formation and stability of the ferryl intermediate. J Biol Chem. 1999;274:2029–2037. - PubMed
-
- Allentoff AJ. Bolton JL. Wilks A. Thompson JA. Ortiz de Montellano PR. Heterolytic versus homolytic peroxide bond cleavage by sperm whale myoglobin and myoglobin mutants. J Am Chem Soc. 1992;114:9744–9749.
-
- Andersen HJ. Bertelsen G. Skibsted LH. Salt effect on acid-catalyzed autoxidation of oxymyoglobin. Acta Chemica Scand A. 1988;42:226–236.
-
- Andersen HJ. Skibsted LH. Kinetics and mechanism of thermal oxidation and photooxidation of nitrosylmyoglobin in aqueous solution. J Agric Food Chem. 1992;40:1741–1750.
-
- Antoni E. Brunoni M. Hemoglobin and Myoglobin in Their Reactions with Ligands. Amsterdam, The Netherlands: North-Holland Publ Co; 1971.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

