Role of reactive oxygen species-mediated signaling in aging
- PMID: 22901002
- PMCID: PMC3791051
- DOI: 10.1089/ars.2012.4891
Role of reactive oxygen species-mediated signaling in aging
Abstract
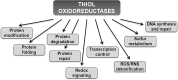
Significance: Redox biology is a rapidly developing area of research due to the recent evidence for general importance of redox control for numerous cellular functions under both physiological and pathophysiological conditions. Understanding of redox homeostasis is particularly relevant to the understanding of the aging process. The link between reactive oxygen species (ROS) and accumulation of age-associated oxidative damage to macromolecules is well established, but remains controversial and applies only to a subset of experimental models. In addition, recent studies show that ROS may function as signaling molecules and that dysregulation of this process may also be linked to aging.
Recent advances: Many protein factors and pathways that control ROS production and scavenging, as well as those that regulate cellular redox homeostasis, have been identified. However, much less is known about the mechanisms by which redox signaling pathways influence longevity. In this review, we discuss recent advances in the understanding of the molecular basis for the role of redox signaling in aging.
Critical issues: Recent studies allowed identification of previously uncharacterized redox components and revealed complexity of redox signaling pathways. It would be important to identify functions of these components and elucidate how distinct redox pathways are integrated with each other to maintain homeostatic balance.
Future directions: Further characterization of processes that coordinate redox signaling, redox homeostasis, and stress response pathways should allow researchers to dissect how their dysregulation contributes to aging and pathogenesis of various age-related diseases, such as diabetes, cancer and neurodegeneration.
Figures






References
-
- Bienert GP. Moller AL. Kristiansen KA. Schulz A. Moller IM. Schjoerring JK. Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. - PubMed
-
- Biteau B. Labarre J. Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. - PubMed
-
- Bozonet SM. Findlay VJ. Day AM. Cameron J. Veal EA. Morgan BA. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J Biol Chem. 2005;280:23319–23327. - PubMed
-
- Brunelle JK. Bell EL. Quesada NM. Vercauteren K. Tiranti V. Zeviani M. Scarpulla RC. Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

