Probing the peripheral site of human butyrylcholinesterase
- PMID: 22901043
- PMCID: PMC3438789
- DOI: 10.1021/bi300955k
Probing the peripheral site of human butyrylcholinesterase
Abstract
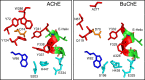
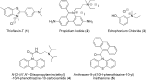
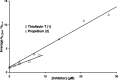
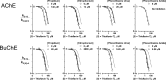
Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) catalyze the hydrolysis of the neurotransmitter acetylcholine and, thereby, function as coregulators of cholinergic neurotransmission. For both enzymes, hydrolysis takes place near the bottom of a 20 Å deep active site gorge. A number of amino acid residues within the gorge have been identified as important in facilitating efficient catalysis and inhibitor binding. Of particular interest is the catalytic triad, consisting of serine, histidine, and glutamate residues, that mediates hydrolysis. Another site influencing the catalytic process is located above the catalytic triad toward the periphery of the active site gorge. This peripheral site (P-site) contains a number of aromatic amino acid residues as well as an aspartate residue that is able to interact with cationic substrates and guide them down the gorge to the catalytic triad. In human AChE, certain aryl residues in the vicinity of the anionic aspartate residue (D74), such as W286, have been implicated in ligand binding and have therefore been considered part of the P-site of the enzyme. The present study was undertaken to explore the P-site of human BuChE and determine whether, like AChE, aromatic side chains near the peripheral aspartate (D70) of this enzyme contribute to ligand binding. Results obtained, utilizing inhibitor competition studies and BuChE mutant species, indicate the participation of aryl residues (F329 and Y332) in the E-helix component of the BuChE active site gorge, along with the anionic aspartate residue (D70), in binding ligands to the P-site of the enzyme.
Figures
References
-
- Silver A. (1974) The Biology of Cholinesterases, Elsevier, Amsterdam.
-
- Sussman J. L.; Harel M.; Frolow F.; Oefner C.; Goldman A.; Toker L.; Silman I. (1991) Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science 253, 872–879. - PubMed
-
- Nicolet Y.; Lockridge O.; Masson P.; Fontecilla-Camps J. C.; Nachon F. (2003) Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 278, 41141–41147. - PubMed
-
- Szegletes T.; Mallender W. D.; Rosenberry T. L. (1998) Nonequilibrium analysis alters the mechanistic interpretation of inhibition of acetylcholinesterase by peripheral site ligands. Biochemistry 37, 4206–4216. - PubMed
-
- Johnson J. L.; Cusack B.; Davies M. P.; Fauq A.; Rosenberry T. L. (2003) Unmasking tandem site interaction in human acetylcholinesterase. Substrate activation with a cationic acetanilide substrate. Biochemistry 42, 5438–5452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

