Mitochondria-mediated energy adaption in cancer: the H(+)-ATP synthase-geared switch of metabolism in human tumors
- PMID: 22901241
- PMCID: PMC3691914
- DOI: 10.1089/ars.2012.4883
Mitochondria-mediated energy adaption in cancer: the H(+)-ATP synthase-geared switch of metabolism in human tumors
Abstract
Significance: Since the signing of the National Cancer Act in 1971, cancer still remains a major cause of death despite significant progresses made in understanding the biology and treatment of the disease. After many years of ostracism, the peculiar energy metabolism of tumors has been recognized as an additional phenotypic trait of the cancer cell.
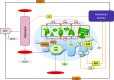
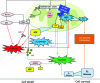
Recent advances: While the enhanced aerobic glycolysis of carcinomas has already been translated to bedside for precise tumor imaging and staging of cancer patients, accepting that an impaired bioenergetic function of mitochondria is pivotal to understand energy metabolism of tumors and in its progression is debated. However, mitochondrial bioenergetics and cell death are tightly connected.
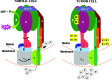
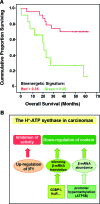
Critical issues: Recent clinical findings indicate that H(+)-ATP synthase, a core component of mitochondrial oxidative phosphorylation, is repressed at both the protein and activity levels in human carcinomas. This review summarizes the relevance that mitochondrial function has to understand energy metabolism of tumors and explores the connection between the bioenergetic function of the organelle and the activity of mitochondria as tumor suppressors.
Future directions: The reversible nature of energy metabolism in tumors highlights the relevance that the microenvironment has for tumor progression. Moreover, the stimulation of mitochondrial activity or the inhibition of glycolysis suppresses tumor growth. Future research should elucidate the mechanisms promoting the silencing of oxidative phosphorylation in carcinomas. The aim is the development of new therapeutic strategies tackling energy metabolism to eradicate tumors or at least, to maintain tumor dormancy and transform cancer into a chronic disease.
Figures

Similar articles
-
[Research on relevance between mitochondrial ATP synthase and malignant tumor].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2014 Jun;31(3):714-7. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2014. PMID: 25219263 Review. Chinese.
-
Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype.J Biol Chem. 2010 Aug 13;285(33):25308-13. doi: 10.1074/jbc.M110.146480. Epub 2010 Jun 9. J Biol Chem. 2010. PMID: 20538613 Free PMC article.
-
Loss of the mitochondrial bioenergetic capacity underlies the glucose avidity of carcinomas.Cancer Res. 2007 Oct 1;67(19):9013-7. doi: 10.1158/0008-5472.CAN-07-1678. Cancer Res. 2007. PMID: 17909002
-
Post-transcriptional regulation of the mitochondrial H(+)-ATP synthase: a key regulator of the metabolic phenotype in cancer.Biochim Biophys Acta. 2011 Jun;1807(6):543-51. doi: 10.1016/j.bbabio.2010.10.016. Epub 2010 Oct 27. Biochim Biophys Acta. 2011. PMID: 21035425 Review.
-
Reprogramming Oxidative Phosphorylation in Cancer: A Role for RNA-Binding Proteins.Antioxid Redox Signal. 2020 Nov 1;33(13):927-945. doi: 10.1089/ars.2019.7988. Epub 2020 Jan 30. Antioxid Redox Signal. 2020. PMID: 31910046 Review.
Cited by
-
A Review of the Inhibition of the Mitochondrial ATP Synthase by IF1 in vivo: Reprogramming Energy Metabolism and Inducing Mitohormesis.Front Physiol. 2018 Sep 19;9:1322. doi: 10.3389/fphys.2018.01322. eCollection 2018. Front Physiol. 2018. PMID: 30283362 Free PMC article. Review.
-
The Dual Function of Reactive Oxygen/Nitrogen Species in Bioenergetics and Cell Death: The Role of ATP Synthase.Oxid Med Cell Longev. 2016;2016:3869610. doi: 10.1155/2016/3869610. Epub 2016 Mar 10. Oxid Med Cell Longev. 2016. PMID: 27034734 Free PMC article. Review.
-
Dysfunctional oxidative phosphorylation shunts branched-chain amino acid catabolism onto lipogenesis in skeletal muscle.EMBO J. 2020 Jul 15;39(14):e103812. doi: 10.15252/embj.2019103812. Epub 2020 Jun 3. EMBO J. 2020. PMID: 32488939 Free PMC article.
-
A naturally occurring polyacetylene isolated from carrots promotes health and delays signatures of aging.Nat Commun. 2023 Dec 8;14(1):8142. doi: 10.1038/s41467-023-43672-7. Nat Commun. 2023. PMID: 38065964 Free PMC article.
-
Differential Proteomics Analysis of the Subcutaneous Connective Tissues in Alcian Blue Tracks along Conception Vessel and Adjacent Nonmeridian in Rats.Evid Based Complement Alternat Med. 2021 May 4;2021:5550694. doi: 10.1155/2021/5550694. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34035822 Free PMC article.
References
-
- Alavian KN. Li H. Collis L. Bonanni L. Zeng L. Sacchetti S. Lazrove E. Nabili P. Flaherty B. Graham M. Chen Y. Messerli SM. Mariggio MA. Rahner C. McNay E. Shore GC. Smith PJ. Hardwick JM. Jonas EA. Bcl-x(L) regulates metabolic efficiency of neurons through interaction with the mitochondrial F(1)F(O) ATP synthase. Nat Cell Biol. 2011;13:1224–1233. - PMC - PubMed
-
- Aldea M. Clofent J. Nunez de Arenas C. Chamorro M. Velasco M. Berrendero JR. Navarro C. Cuezva JM. Reverse phase protein microarrays quantify and validate the bioenergetic signature as biomarker in colorectal cancer. Cancer Lett. 2011;311:210–218. - PubMed
-
- Arismendi-Morillo G. Electron microscopy morphology of the mitochondrial network in gliomas and their vascular microenvironment. Biochim Biophys Acta. 2011;1807:602–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

