Protein markers of cancer-associated fibroblasts and tumor-initiating cells reveal subpopulations in freshly isolated ovarian cancer ascites
- PMID: 22901285
- PMCID: PMC3517779
- DOI: 10.1186/1471-2407-12-359
Protein markers of cancer-associated fibroblasts and tumor-initiating cells reveal subpopulations in freshly isolated ovarian cancer ascites
Abstract
Background: In ovarian cancer, massive intraperitoneal dissemination is due to exfoliated tumor cells in ascites. Tumor-initiating cells (TICs or cancer stem cells) and cells showing epithelial-mesenchymal-transition (EMT) are particularly implicated. Spontaneous spherical cell aggregates are sometimes observed, but although similar to those formed by TICs in vitro, their significance is unclear.
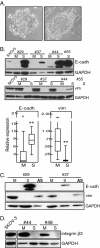
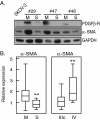
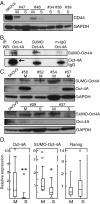
Methods: Cells freshly isolated from malignant ascites were separated into sphere samples (S-type samples, n=9) and monolayer-forming single-cell suspensions (M-type, n=18). Using western blot, these were then compared for expression of protein markers of EMT, TIC, and of cancer-associated fibroblasts (CAFs).
Results: S-type cells differed significantly from M-type by expressing high levels of E-cadherin and no or little vimentin, integrin-β3 or stem cell transcription factor Oct-4A. By contrast, M-type samples were enriched for CD44, Oct-4A and for CAF markers. Independently of M- and S-type, there was a strong correlation between TIC markers Nanog and EpCAM. The CAF marker α-SMA correlated with clinical stage IV. This is the first report on CAF markers in malignant ascites and on SUMOylation of Oct-4A in ovarian cancer.
Conclusions: In addition to demonstrating potentially high levels of TICs in ascites, the results suggest that the S-type population is the less tumorigenic one. Nanog(high)/EpCAM(high) samples represent a TIC subset which may be either M- or S-type, and which is separate from the CD44(high)/Oct-4A(high) subset observed only in M-type samples. This demonstrates a heterogeneity in TIC populations in vivo which has practical implications for TIC isolation based on cell sorting. The biological heterogeneity will need to be addressed in future therapeutical strategies.
Figures
References
-
- Burleson KM, Hansen LK, Skubitz AP. Ovarian carcinoma spheroids disaggregate on type I collagen and invade live human mesothelial cell monolayers. Clin Exp Metastasis. 2004;21(8):685–697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

