Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions
- PMID: 22901435
- PMCID: PMC3516706
- DOI: 10.1016/j.jaut.2012.07.006
Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions
Abstract
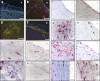
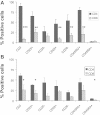
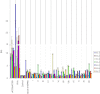
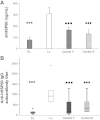
Atherosclerosis is a multifactorial chronic inflammatory disease characterized by the presence of T-cells, macrophages, and dendritic cells in the arterial intima. Classical risk factors lead to over-expression of stress proteins, especially heat shock protein 60 (HSP60). HSP60 on the surface of arterial endothelial cells (ECs) then becomes a target for pre-existing adaptive anti-HSP60 immunity resulting in infiltration of the intima by mononuclear cells. In the present study, T-cells derived from early, clinically still inapparent human atherosclerotic lesions were analyzed phenotypically and for their reactivity against HSP60 and HSP60-derived peptides. HSP60 was detected in ECs and CD40- and HLA Class II-positive cells within the intima. Effector memory CD4(+) T-cells producing high amounts of interferon-γ and low levels of interleukin-4 were the dominant subpopulation. T-cells derived from late lesions displayed a more restricted T-cell receptor repertoire to HSP60-derived peptides than those isolated from early lesions. Increased levels of soluble HSP60 and circulating anti-human HSP60 autoantibodies were found in donors with late but not early lesions. This is the first functional study of T-cells derived from early human atherosclerotic lesions that supports the previously proposed concept that HSP60-reactive T-cells initiate atherosclerosis by recognition of atherogenic HSP60 epitopes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Boring L., Gosling J., Cleary M., Charo I.F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. - PubMed
-
- Gu L., Okada Y., Clinton S.K., Gerard C., Sukhova G.K., Libby P. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. - PubMed
-
- Young D.B. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol. 1992;4:396–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

