Clinical outcomes of neonatal onset proximal versus distal urea cycle disorders do not differ
- PMID: 22901741
- PMCID: PMC4440324
- DOI: 10.1016/j.jpeds.2012.06.065
Clinical outcomes of neonatal onset proximal versus distal urea cycle disorders do not differ
Abstract
Objective: To compare the clinical course and outcome of patients diagnosed with one of 4 neonatal-onset urea cycle disorders (UCDs): deficiency of carbamyl phosphate synthase 1 (CPSD), ornithine transcarbamylase (OTCD), argininosuccinate synthase (ASD), or argininosuccinate lyase (ALD).
Study design: Clinical, biochemical, and neuropsychological data from 103 subjects with neonatal-onset UCDs were derived from the Longitudinal Study of Urea Cycle Disorders, an observational protocol of the Urea Cycle Disorders Consortium, one of the Rare Disease Clinical Research Networks.
Results: Some 88% of the subjects presented clinically by age 7 days. Peak ammonia level was 963 μM in patients with proximal UCDs (CPSD or OTCD), compared with 589 μM in ASD and 573 μM in ALD. Roughly 25% of subjects with CPSD or OTCD, 18% of those with ASD, and 67% of those with ALD had a "honeymoon period," defined as the time interval from discharge from initial admission to subsequent admission for hyperammonemia, greater than 1 year. The proportion of patients with a poor outcome (IQ/Developmental Quotient <70) was greatest in ALD (68%), followed by ASD (54%) and CPSD/OTCD (47%). This trend was not significant, but was observed in both patients aged <4 years and those aged ≥ 4 years. Poor cognitive outcome was not correlated with peak ammonia level or duration of initial admission.
Conclusion: Neurocognitive outcomes do not differ between patients with proximal UCDs and those with distal UCDs. Factors other than hyperammonemia may contribute to poor neurocognitive outcome in the distal UCDs.
Trial registration: ClinicalTrials.gov NCT00237315.
Copyright © 2013 Mosby, Inc. All rights reserved.
Figures

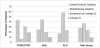
Profound/Severe Range of Disability: Subject was not testable by traditional IQ testing for their age range (i.e., WPPSI for 3–5 year olds and WASI for 6 years and older). Bayley Scales were instead administered to derive a developmental quotient (DQ).
Mild-Moderate Range of Disability: FSIQ Score of 45–69. If no FSIQ was available, Verbal Intelligence Quotient (VIQ) or Performance Intelligence Quotient (PIQ) was instead used to determine categorization.
Low Average/Borderline Functioning: FSIQ Score of 70–89. If no FSIQ was available, VIQ or PIQ was instead used to determine categorization.
Broadly Average: FSIQ Score of 90–109. If no FSIQ is available, VIQ or PIQ was instead used to determine categorization.
Above Average: FSIQ ≥ 110. No subject met this criterion.
References
-
- Summar M, Tuchman M. Proceedings of a consensus conference for the management of patients with urea cycle disorders. J Pediatr. 2001;138:S6–10. - PubMed
-
- Batshaw ML. Hyperammonemia. Curr Probl Pediatr. 1984;14:1–69. - PubMed
-
- Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984;310:1500–5. - PubMed
-
- Maestri NE, Hauser ER, Bartholomew D, Brusilow SW. Prospective treatment of urea cycle disorders. J Pediatr. 1991;119:923–8. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

