Cohesin's DNA exit gate is distinct from its entrance gate and is regulated by acetylation
- PMID: 22901742
- PMCID: PMC3485559
- DOI: 10.1016/j.cell.2012.07.028
Cohesin's DNA exit gate is distinct from its entrance gate and is regulated by acetylation
Abstract
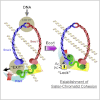
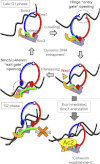
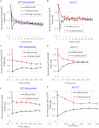
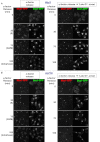
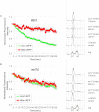
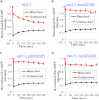
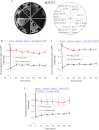
Sister chromatid cohesion is mediated by entrapment of sister DNAs by a tripartite ring composed of cohesin's Smc1, Smc3, and α-kleisin subunits. Cohesion requires acetylation of Smc3 by Eco1, whose role is to counteract an inhibitory (antiestablishment) activity associated with cohesin's Wapl subunit. We show that mutations abrogating antiestablishment activity also reduce turnover of cohesin on pericentric chromatin. Our results reveal a "releasing" activity inherent to cohesin complexes transiently associated with Wapl that catalyzes their dissociation from chromosomes. Fusion of Smc3's nucleotide binding domain to α-kleisin's N-terminal domain also reduces cohesin turnover within pericentric chromatin and permits establishment of Wapl-resistant cohesion in the absence of Eco1. We suggest that releasing activity opens the Smc3/α-kleisin interface, creating a DNA exit gate distinct from its proposed entry gate at the Smc1/3 interface. According to this notion, the function of Smc3 acetylation is to block its dissociation from α-kleisin. The functional implications of regulated ring opening are discussed.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





References
-
- Arumugam P., Gruber S., Tanaka K., Haering C.H., Mechtler K., Nasmyth K. ATP hydrolysis is required for cohesin’s association with chromosomes. Curr. Biol. 2003;13:1941–1953. - PubMed
-
- Ciosk R., Shirayama M., Shevchenko A., Tanaka T., Toth A., Shevchenko A., Nasmyth K. Cohesin’s binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol. Cell. 2000;5:243–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

