A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma
- PMID: 22902362
- PMCID: PMC3912579
- DOI: 10.1038/leu.2012.236
A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma
Abstract
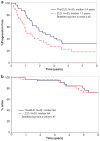
Patients with asymptomatic (smoldering) multiple myeloma (AMM) have a high risk of transformation to active multiple myeloma (MM). Bisphosphonates such as zoledronic acid (ZLD) reduce skeletal events in MM and the immunomodulatory agent thalidomide (Thal) has proven effectiveness in active MM. We hypothesized that treatment with Thal and ZLD would prolong the time to progression (TTP) to MM over ZLD alone. Eligible patients had asymptomatic MM and all patients received ZLD 4 mg intravenous monthly; the treatment arm also received Thal 200 mg per day. The TTP was superior for Thal/ZLD (n=35) patients compared with ZLD alone (n=33); median TTP of 2.4 years (95% confidence interval (CI): 1.4-3.6) versus 1.2 years (95% CI: 0.7-2.5) (hazard ratio (HR), 2.05; 95% CI: 1.1-3.8; P-value: 0.02). At 1 year, 86% of Thal/ZLD patients were progression free compared with 55% on ZLD alone (P=0.0048). The overall response rate after year 1 was 37% for Thal/ZLD with a median duration of response of 3.3 years (95% CI: 1.1-NA); there were no confirmed responses to ZLD alone (P=0.0004). The addition of Thal to standard ZLD produces anti-tumor responses whereas ZLD alone does not. Thal/ZLD also prolongs TTP from AMM to MM. This study provides the rationale for further studies in patients with AMM to delay chemotherapy.
Trial registration: ClinicalTrials.gov NCT00432458.
Conflict of interest statement
The study drugs thalidomide and Zometa (zoledronic acid) were provided by Celgene Pharmaceuticals and Novartis Oncology, respectively. Dr Witzig, Rajkumar, Lacy, Dispenzieri, Hassoun all have received research funding from Celgene for clinical trials. Dr Witzig has received research funding from Novartis for other clinical trials. Dr Gertz has received honoraria for lectures from Celgene. The remaining authors declare no conflicts of interest.
Figures


References
-
- Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. - PubMed
-
- Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. - PubMed
-
- Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

