The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation
- PMID: 22902403
- PMCID: PMC3463940
- DOI: 10.1016/j.celrep.2012.07.007
The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation
Abstract
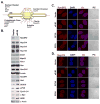
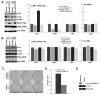
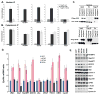
The eukaryotic translation initiation factor eIF4E is a potent oncogene that promotes the nuclear export and translation of specific transcripts. Here, we have discovered that eIF4E alters the cytoplasmic face of the nuclear pore complex (NPC), which leads to enhanced mRNA export of eIF4E target mRNAs. Specifically, eIF4E substantially reduces the major component of the cytoplasmic fibrils of the NPC, RanBP2, relocalizes an associated nucleoporin, Nup214, and elevates RanBP1 and the RNA export factors, Gle1 and DDX19. Genetic or pharmacological inhibition of eIF4E impedes these effects. RanBP2 overexpression specifically inhibits the eIF4E mRNA export pathway and impairs oncogenic transformation by eIF4E. The RanBP2 cytoplasmic fibrils most likely slow the release and/or recycling of critical export factors to the nucleus. eIF4E overcomes this inhibitory mechanism by indirectly reducing levels of RanBP2. More generally, these results suggest that reprogramming the NPC is a means by which oncogenes can harness the proliferative capacity of the cell.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, Caplan S, Leber B, Roy DC, Miller WH, Jr, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–260. - PubMed
-
- Boer JM, van Deursen JM, Croes HJ, Fransen JA, Grosveld GC. The nucleoporin CAN/Nup214 binds to both the cytoplasmic and the nucleoplasmic sides of the nuclear pore complex in overexpressing cells. Exp Cell Res. 1997;232:182–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

