Impact of individual proliferating cell nuclear antigen-DNA contacts on clamp loading and function on DNA
- PMID: 22902629
- PMCID: PMC3471718
- DOI: 10.1074/jbc.M112.399071
Impact of individual proliferating cell nuclear antigen-DNA contacts on clamp loading and function on DNA
Abstract
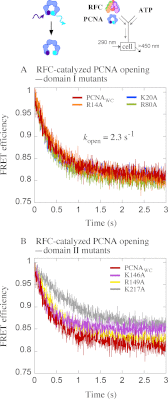
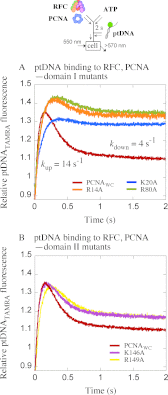
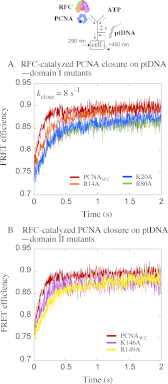
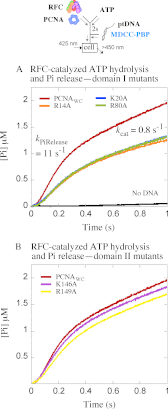
Ring-shaped clamp proteins encircle DNA and affect the work of many proteins, notably processive replication by DNA polymerases. Crystal structures of clamps show several cationic residues inside the ring, and in a co-crystal of Escherichia coli β clamp-DNA, they directly contact the tilted duplex passing through (Georgescu, R. E., Kim, S. S., Yurieva, O., Kuriyan, J., Kong, X. P., and O'Donnell, M. (2008) Structure of a sliding clamp on DNA. Cell 132, 43-54). To investigate the role of these contacts in reactions involving circular clamps, we examined single arginine/lysine mutants of Saccharomyces cerevisiae proliferating cell nuclear antigen (PCNA) in replication factor C (RFC)-catalyzed loading of the clamp onto primer template DNA (ptDNA). Previous kinetic analysis has shown that ptDNA entry inside an ATP-activated RFC-PCNA complex accelerates clamp opening and ATP hydrolysis, which is followed by slow PCNA closure around DNA and product dissociation. Here we directly measured multiple steps in the reaction (PCNA opening, ptDNA binding, PCNA closure, phosphate release, and complex dissociation) to determine whether mutation of PCNA residues Arg-14, Lys-20, Arg-80, Lys-146, Arg-149, or Lys-217 to alanine affects the reaction mechanism. Contrary to earlier steady state analysis of these mutants (McNally, R., Bowman, G. D., Goedken, E. R., O'Donnell, M., and Kuriyan, J. (2010) Analysis of the role of PCNA-DNA contacts during clamp loading. BMC Struct. Biol. 10, 3), our pre-steady state data show that loss of single cationic residues can alter the rates of all DNA-linked steps in the reaction, as well as movement of PCNA on DNA. These results explain an earlier finding that individual arginines and lysines inside human PCNA are essential for polymerase δ processivity (Fukuda, K., Morioka, H., Imajou, S., Ikeda, S., Ohtsuka, E., and Tsurimoto, T. (1995) Structure-function relationship of the eukaryotic DNA replication factor, proliferating cell nuclear antigen. J. Biol. Chem. 270, 22527-22534). Mutations in the N-terminal domain have greater impact than in the C-terminal domain, indicating a positional bias in PCNA-DNA contacts that can influence its functions on DNA.
Figures



References
-
- Fukuda K., Morioka H., Imajou S., Ikeda S., Ohtsuka E., Tsurimoto T. (1995) Structure-function relationship of the eukaryotic DNA replication factor, proliferating cell nuclear antigen. J. Biol. Chem. 270, 22527–22534 - PubMed
-
- Johnson A., O'Donnell M. (2005) Cellular DNA replicases. Components and dynamics at the replication fork. Annu. Rev. Biochem. 74, 283–315 - PubMed
-
- Moldovan G. L., Pfander B., Jentsch S. (2007) PCNA, the maestro of the replication fork. Cell 129, 665–679 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

