Disease Progression in HIV-1-Infected Viremic Controllers
- PMID: 22902723
- PMCID: PMC3736311
- DOI: 10.1097/QAI.0b013e318269c414
Disease Progression in HIV-1-Infected Viremic Controllers
Abstract
Background: The mechanism of CD4 T-cell decline in HIV-1 infection is unclear, but the association with plasma viral RNA load suggests viral replication is involved. Indeed, viremic controller patients with low viral RNA loads typically maintain high CD4 T-cell counts. Within a local cohort of 86 viremic controllers, we identify a subgroup (18 "discord controllers") with low CD4 T-cell counts that present clinical uncertainty. The underlying mechanism accounting for CD4 T-cell decline in the face of low or undetectable plasma (RNA) viral load remains unresolved. The objective of this study was to investigate the viral and host immune system dynamics in discord controllers by measuring cellular HIV-1 DNA load, T-cell populations, and T-cell activation markers.
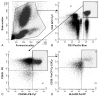
Methods: We compared discord controllers (viral RNA load <2000 copies/mL, <450 CD4 T-cells/mm) with typical controllers (viral RNA load <2000 copies/mL, >450 CD4 T-cells/mm) and progressors (viral RNA load >10,000 copies/mL, <450 CD4 T-cells/mm). We quantified CD4/CD8 naive/central memory/effector memory subsets (CD45RA/RO ± CD62L), activation levels (CD38HLA-DR), and HIV-1 DNA load.
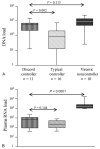
Results: Discord controllers resembled progressors showing high viral DNA load, depletion of naive CD4 T-cells, and higher activation in all CD4 T-cell subsets, compared with typical controllers. They were similar to typical controllers with lower CD8 T-cell activation compared with progressors.
Conclusions: Our data are consistent with a relationship between CD4 T-cell activation and disease progression. HIV-1 DNA load may be a better marker of viral replication and disease progression than viral RNA load. Lower level CD8 T-cell activation correlates with low viral RNA load but not with disease progression or viral DNA load.
Figures


References
-
- Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. - PubMed
-
- Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. - PubMed
-
- Rodriguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–1506. - PubMed
-
- Deeks SG, Kitchen CMR, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. - PubMed
-
- Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

