Portal hypertension in children and young adults with biliary atresia
- PMID: 22903006
- PMCID: PMC3483444
- DOI: 10.1097/MPG.0b013e31826eb0cf
Portal hypertension in children and young adults with biliary atresia
Abstract
Objective: Biliary atresia (BA) frequently results in portal hypertension (PHT), complications of which lead to significant morbidity and mortality. The Childhood Liver Disease Research and Education Network was used to perform a cross-sectional multicentered analysis of PHT in children with BA.
Methods: Subjects with BA receiving medical management at a Childhood Liver Disease Research and Education Network site were enrolled. A priori, clinically evident PHT was defined as "definite" when there was either history of a complication of PHT or clinical findings consistent with PHT (both splenomegaly and thrombocytopenia). PHT was denoted as "possible" if one of the findings was present in the absence of a complication, whereas PHT was "absent" if none of the criteria were met.
Results: A total of 163 subjects were enrolled between May 2006 and December 2009. At baseline, definite PHT was present in 49%, possible in 17%, and absent in 34% of subjects. Demographics, growth, and anthropometrics were similar amongst the 3 PHT categories. Alanine aminotransferase, γ-glutamyl transpeptidase, and sodium levels were similar, whereas there were significant differences in aspartate aminotransferase (AST), AST/alanine aminotransferase, albumin, total bilirubin, prothrombin time, white blood cell count, platelet count, and AST/platelet count between definite and absent PHT. Thirty-four percent of those with definite PHT had either prothrombin time >15 seconds or albumin <3 g/dL.
Conclusions: Clinically definable PHT is present in two-thirds of North American long-term BA survivors with their native livers. The presence of PHT is associated with measures of hepatic injury and dysfunction, although in this selected cohort, the degree of hepatic dysfunction is relatively mild and growth is preserved.
Trial registration: ClinicalTrials.gov NCT00061828.
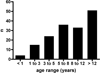
Figures







References
-
- Shneider BL, Mazariegos GV. Biliary atresia: a transplant perspective. Liver Transpl. 2007;13:1482–1495. - PubMed
-
- Duche M, Fabre M, Kretzschmar B, Serinet MO, Gauthier F, Chardot C. Prognostic value of portal pressure at the time of Kasai operation in patients with biliary atresia. J Pediatr Gastroenterol Nutr. 2006;43:640–645. - PubMed
-
- Laurent J, Gauthier F, Bernard O, Hadchouel M, Odievre M, Valayer J, Alagille D. Long-term outcome after surgery for biliary atresia. Study of 40 patients surviving for more than 10 years. Gastroenterology. 1990;99:1793–1797. - PubMed
-
- Davenport M, Kerkar N, Mieli-Vergani G, Mowat AP, Howard ER. Biliary atresia: the King's College Hospital experience (1974–1995) J Pediatr Surg. 1997;32:479–485. - PubMed
-
- Stringer M, Howard E, Mowat A. Endoscopic sclerotherapy in the management of esophageal varices in 61 children with biliary atresia. J. Pediatr. Surg. 1989;24:438–492. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- DK 62470/DK/NIDDK NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- U01 DK062452/DK/NIDDK NIH HHS/United States
- U01 DK062466/DK/NIDDK NIH HHS/United States
- UL1RR024131/RR/NCRR NIH HHS/United States
- UL1RR025780/RR/NCRR NIH HHS/United States
- 5M01 RR00069/RR/NCRR NIH HHS/United States
- U01 DK062445/DK/NIDDK NIH HHS/United States
- U24 DK062456/DK/NIDDK NIH HHS/United States
- UL1RR025005/RR/NCRR NIH HHS/United States
- U01 DK062481/DK/NIDDK NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U01 DK084585/DK/NIDDK NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- DK 62445/DK/NIDDK NIH HHS/United States
- DK 62452/DK/NIDDK NIH HHS/United States
- UL1RR025741/RR/NCRR NIH HHS/United States
- DK 62530/DK/NIDDK NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- DK 62497/DK/NIDDK NIH HHS/United States
- DK 62456/DK/NIDDK NIH HHS/United States
- U01 DK062453/DK/NIDDK NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- DK 62436/DK/NIDDK NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- DK 62453/DK/NIDDK NIH HHS/United States
- U01 DK062436/DK/NIDDK NIH HHS/United States
- UL1RR024153/RR/NCRR NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- DK 62500/DK/NIDDK NIH HHS/United States
- U01 DK062456/DK/NIDDK NIH HHS/United States
- TL1 RR029877/RR/NCRR NIH HHS/United States
- UL1RR024134/RR/NCRR NIH HHS/United States
- DK 84585/DK/NIDDK NIH HHS/United States
- DK 62481/DK/NIDDK NIH HHS/United States
- U01 DK062500/DK/NIDDK NIH HHS/United States
- U01 DK062497/DK/NIDDK NIH HHS/United States
- DK 62466/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

