The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells
- PMID: 22903229
- PMCID: PMC3471131
- DOI: 10.1002/eji.201242370
The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells
Abstract
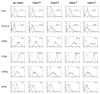
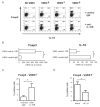
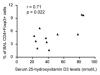
1α,25-Dihydroxyvitamin D3 (1α25VitD3) has potent immunomodulatory properties. We have previously demonstrated that 1α25VitD3 promotes human and murine IL-10-secreting CD4(+) T cells. Because of the clinical relevance of this observation, we characterized these cells further and investigated their relationship with Foxp3(+) regulatory T (Treg) cells. 1α25VitD3 increased the frequency of both Foxp3(+) and IL-10(+) CD4(+) T cells in vitro. However, Foxp3 was increased at high concentrations of 1α25VitD3 and IL-10 at more moderate levels, with little coexpression of these molecules. The Foxp3(+) and IL-10(+) T-cell populations showed comparable suppressive activity. We demonstrate that the enhancement of Foxp3 expression by 1α25VitD3 is impaired by IL-10. 1α25VitD3 enables the selective expansion of Foxp3(+) Treg cells over their Foxp3(-) T-cell counterparts. Equally, 1α25VitD3 maintains Foxp3(+) expression by sorted populations of human and murine Treg cells upon in vitro culture. A positive in vivo correlation between vitamin D status and CD4(+) Foxp3(+) T cells in the airways was observed in a severe pediatric asthma cohort, supporting the in vitro observations. In summary, we provide evidence that 1α25VitD3 enhances the frequency of both IL-10(+) and Foxp3(+) Treg cells. In a translational setting, these data suggest that 1α25VitD3, over a broad concentration range, will be effective in enhancing the frequency of Treg cells.
© 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

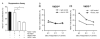

References
-
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010;10:490–500. - PubMed
-
- Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009;70:345–352. - PubMed
-
- Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. - PubMed
-
- Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur. J. Immunol. 2009;39:3147–3159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

