Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation
- PMID: 22903385
- PMCID: PMC11028421
- DOI: 10.1007/s00262-012-1334-1
Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation
Abstract
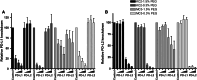
Dendritic cell (DC)-based vaccination boosting antigen-specific immunity is being explored for the treatment of cancer and chronic viral infections. Although DC-based immunotherapy can induce immunological responses, its clinical benefit has been limited, indicating that further improvement of DC vaccine potency is essential. In this study, we explored the generation of a clinical-grade applicable DC vaccine with improved immunogenic potential by combining PD-1 ligand siRNA and target antigen mRNA delivery. We demonstrated that PD-L1 and PD-L2 siRNA delivery using DLin-KC2-DMA-containing lipid nanoparticles (LNP) mediated efficient and specific knockdown of PD-L expression on human monocyte-derived DC. The established siRNA-LNP transfection method did not affect DC phenotype or migratory capacity and resulted in acceptable DC viability. Furthermore, we showed that siRNA-LNP transfection can be successfully combined with both target antigen peptide loading and mRNA electroporation. Finally, we demonstrated that these PD-L-silenced DC loaded with antigen mRNA superiorly boost ex vivo antigen-specific CD8(+) T cell responses from transplanted cancer patients. Together, these findings indicate that our PD-L siRNA-LNP-modified DC are attractive cells for clinical-grade production and in vivo application to induce and boost immune responses not only in transplanted cancer patients, but likely also in other settings.
Conflict of interest statement
Tatiana I. Novobrantseva, Jamie Wong, Stuart Milstein, Hila Epstein-Barash and Ju Liu are employees of Alnylam Pharmaceuticals. The other authors have no conflicting financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

