Novel pyrophosphate-forming acetate kinase from the protist Entamoeba histolytica
- PMID: 22903977
- PMCID: PMC3485911
- DOI: 10.1128/EC.00169-12
Novel pyrophosphate-forming acetate kinase from the protist Entamoeba histolytica
Abstract
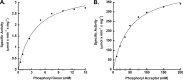
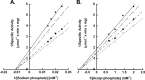
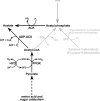
Acetate kinase (ACK) catalyzes the reversible synthesis of acetyl phosphate by transfer of the γ-phosphate of ATP to acetate. Here we report the first biochemical and kinetic characterization of a eukaryotic ACK, that from the protist Entamoeba histolytica. Our characterization revealed that this protist ACK is the only known member of the ASKHA structural superfamily, which includes acetate kinase, hexokinase, and other sugar kinases, to utilize inorganic pyrophosphate (PP(i))/inorganic phosphate (P(i)) as the sole phosphoryl donor/acceptor. Detection of ACK activity in E. histolytica cell extracts in the direction of acetate/PP(i) formation but not in the direction of acetyl phosphate/P(i) formation suggests that the physiological direction of the reaction is toward acetate/PP(i) production. Kinetic parameters determined for each direction of the reaction are consistent with this observation. The E. histolytica PP(i)-forming ACK follows a sequential mechanism, supporting a direct in-line phosphoryl transfer mechanism as previously reported for the well-characterized Methanosarcina thermophila ATP-dependent ACK. Characterizations of enzyme variants altered in the putative acetate/acetyl phosphate binding pocket suggested that acetyl phosphate binding is not mediated solely through a hydrophobic interaction but also through the phosphoryl group, as for the M. thermophila ACK. However, there are key differences in the roles of certain active site residues between the two enzymes. The absence of known ACK partner enzymes raises the possibility that ACK is part of a novel pathway in Entamoeba.
Figures

Similar articles
-
Crystal structures of acetate kinases from the eukaryotic pathogens Entamoeba histolytica and Cryptococcus neoformans.J Struct Biol. 2013 Feb;181(2):185-9. doi: 10.1016/j.jsb.2012.11.001. Epub 2012 Nov 16. J Struct Biol. 2013. PMID: 23159802 Free PMC article.
-
Investigation of pyrophosphate versus ATP substrate selection in the Entamoeba histolytica acetate kinase.Sci Rep. 2017 Jul 19;7(1):5912. doi: 10.1038/s41598-017-06156-5. Sci Rep. 2017. PMID: 28724909 Free PMC article.
-
Roles of acetyl-CoA synthetase (ADP-forming) and acetate kinase (PPi-forming) in ATP and PPi supply in Entamoeba histolytica.Biochim Biophys Acta. 2016 Jun;1860(6):1163-72. doi: 10.1016/j.bbagen.2016.02.010. Epub 2016 Feb 23. Biochim Biophys Acta. 2016. PMID: 26922831
-
Characterization of the acetate binding pocket in the Methanosarcina thermophila acetate kinase.J Bacteriol. 2005 Apr;187(7):2386-94. doi: 10.1128/JB.187.7.2386-2394.2005. J Bacteriol. 2005. PMID: 15774882 Free PMC article.
-
Control and regulation of the pyrophosphate-dependent glucose metabolism in Entamoeba histolytica.Mol Biochem Parasitol. 2019 Apr;229:75-87. doi: 10.1016/j.molbiopara.2019.02.002. Epub 2019 Feb 14. Mol Biochem Parasitol. 2019. PMID: 30772421 Review.
Cited by
-
The Role of Acetate Kinase in the Human Parasite Entamoeba histolytica.Parasitologia. 2022 Jun;2(2):147-159. doi: 10.3390/parasitologia2020014. Epub 2022 Jun 16. Parasitologia. 2022. PMID: 36872919 Free PMC article.
-
The Role of Active Site Residues in ATP Binding and Catalysis in the Methanosarcina thermophila Acetate Kinase.Life (Basel). 2015 Mar 12;5(1):861-71. doi: 10.3390/life5010861. Life (Basel). 2015. PMID: 25775277 Free PMC article.
-
Crystal structures of acetate kinases from the eukaryotic pathogens Entamoeba histolytica and Cryptococcus neoformans.J Struct Biol. 2013 Feb;181(2):185-9. doi: 10.1016/j.jsb.2012.11.001. Epub 2012 Nov 16. J Struct Biol. 2013. PMID: 23159802 Free PMC article.
-
Investigation of pyrophosphate versus ATP substrate selection in the Entamoeba histolytica acetate kinase.Sci Rep. 2017 Jul 19;7(1):5912. doi: 10.1038/s41598-017-06156-5. Sci Rep. 2017. PMID: 28724909 Free PMC article.
-
Structural comparisons of phosphoenolpyruvate carboxykinases reveal the evolutionary trajectories of these phosphodiester energy conversion enzymes.J Biol Chem. 2019 Dec 13;294(50):19269-19278. doi: 10.1074/jbc.RA119.010920. Epub 2019 Oct 28. J Biol Chem. 2019. PMID: 31662435 Free PMC article.
References
-
- Aceti DJ, Ferry JG. 1988. Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila: evidence for regulation of synthesis. J. Biol. Chem. 263: 15444– 15448 - PubMed
-
- Anthony RS, Spector LB. 1970. A phosphoenzyme intermediary in acetate kinase action. J. Biol. Chem. 245: 6739– 6741 - PubMed
-
- Anthony RS, Spector LB. 1971. Exchange reactions catalyzed by acetate kinase. J. Biol. Chem. 246: 6129– 6135 - PubMed
-
- Anthony RS, Spector LB. 1972. Phosphorylated acetate kinase. Its isolation and reactivity. J. Biol. Chem. 247: 2120– 2125 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

