Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells
- PMID: 22904098
- PMCID: PMC3605906
- DOI: 10.1093/gerona/gls158
Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells
Abstract
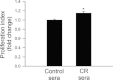
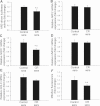
Moderate caloric restriction (CR) without malnutrition increases healthspan in virtually every species studied, including nonhuman primates. In mice, CR exerts significant microvascular protective effects resulting in increased microvascular density in the heart and the brain, which likely contribute to enhanced tolerance to ischemia and improved cardiac performance and cognitive function. Yet, the underlying mechanisms by which CR confer microvascular protection remain elusive. To test the hypothesis that circulating factors triggered by CR regulate endothelial angiogenic capacity, we treated cultured human endothelial cells with sera derived from Macaca mulatta on long-term (over 10 years) CR. Cells treated with sera derived from ad-libitum-fed control monkeys served as controls. We found that factors present in CR sera upregulate vascular endothelial growth factor (VEGF) signaling and stimulate angiogenic processes, including endothelial cell proliferation and formation of capillary-like structures. Treatment with CR sera also tended to increase cellular migration (measured by a wound-healing assay using electric cell-substrate impedance sensing [ECIS] technology) and adhesion to collagen. Collectively, we find that circulating factors induced by CR promote endothelial angiogenic processes, suggesting that increased angiogenesis may be a potential mechanism by which CR improves cardiac function and prevents vascular cognitive impairment.
Figures
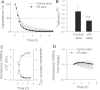



 production in CAECs, assessed using the MitoSOX Red fluorescence method. Data are mean ± SEM. (n = 5 for each group).
production in CAECs, assessed using the MitoSOX Red fluorescence method. Data are mean ± SEM. (n = 5 for each group).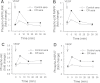

Similar articles
-
Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats.Am J Physiol Heart Circ Physiol. 2014 Aug 1;307(3):H292-306. doi: 10.1152/ajpheart.00307.2014. Epub 2014 Jun 6. Am J Physiol Heart Circ Physiol. 2014. PMID: 24906921 Free PMC article.
-
Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells.J Gerontol A Biol Sci Med Sci. 2015 Jun;70(6):665-74. doi: 10.1093/gerona/glu116. Epub 2014 Aug 18. J Gerontol A Biol Sci Med Sci. 2015. PMID: 25136000 Free PMC article.
-
Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging.J Gerontol A Biol Sci Med Sci. 2012 Aug;67(8):821-9. doi: 10.1093/gerona/glr229. Epub 2012 Jan 4. J Gerontol A Biol Sci Med Sci. 2012. PMID: 22219515 Free PMC article.
-
Caloric restriction and aging in primates: Relevance to humans and possible CR mimetics.Microsc Res Tech. 2002 Nov 15;59(4):335-8. doi: 10.1002/jemt.10214. Microsc Res Tech. 2002. PMID: 12424798 Review.
-
Mechanisms underlying the effects of caloric restriction on hypertension.Biochem Pharmacol. 2022 Jun;200:115035. doi: 10.1016/j.bcp.2022.115035. Epub 2022 Apr 12. Biochem Pharmacol. 2022. PMID: 35427570 Review.
Cited by
-
Aging Exacerbates Pressure-Induced Mitochondrial Oxidative Stress in Mouse Cerebral Arteries.J Gerontol A Biol Sci Med Sci. 2015 Nov;70(11):1355-9. doi: 10.1093/gerona/glu244. Epub 2015 Jan 28. J Gerontol A Biol Sci Med Sci. 2015. PMID: 25631392 Free PMC article.
-
Role of endothelial NAD+ deficiency in age-related vascular dysfunction.Am J Physiol Heart Circ Physiol. 2019 Jun 1;316(6):H1253-H1266. doi: 10.1152/ajpheart.00039.2019. Epub 2019 Mar 15. Am J Physiol Heart Circ Physiol. 2019. PMID: 30875255 Free PMC article. Review.
-
Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease.J Gerontol A Biol Sci Med Sci. 2014 Oct;69(10):1212-26. doi: 10.1093/gerona/glt177. Epub 2013 Nov 22. J Gerontol A Biol Sci Med Sci. 2014. PMID: 24269929 Free PMC article.
-
Do we age because we have mitochondria?Protoplasma. 2014 Jan;251(1):3-23. doi: 10.1007/s00709-013-0515-x. Epub 2013 Jun 22. Protoplasma. 2014. PMID: 23794102 Review.
-
Association of Omnivorous and Vegetarian Diets With Antioxidant Defense Mechanisms in Men.J Am Heart Assoc. 2020 Jun 16;9(12):e015576. doi: 10.1161/JAHA.119.015576. Epub 2020 Jun 9. J Am Heart Assoc. 2020. PMID: 32515251 Free PMC article.
References
-
- Tarnawski AS, Pai R, Tanigawa T, Matysiak-Budnik T, Ahluwalia A. PTEN silencing reverses aging-related impairment of angiogenesis in microvascular endothelial cells. Biochem Biophys Res Commun. 2010;394:291–296 - PubMed
-
- Bach MH, Sadoun E, Reed MJ. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech Ageing Dev. 2005;126:467–473 - PubMed
-
- Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51:1119–1130 - PubMed
-
- Ahluwalia A, Tarnawski AS. Activation of the metabolic sensor-AMP activated protein kinase reverses impairment of angiogenesis in aging myocardial microvascular endothelial cells. Implications for the aging heart. J Physiol Pharmacol. 2011;62:583–587 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AT006526/AT/NCCIH NIH HHS/United States
- R01 AG037000/AG/NIA NIH HHS/United States
- R01 AG040178/AG/NIA NIH HHS/United States
- P01 AG011915/AG/NIA NIH HHS/United States
- P01 AG011370/AG/NIA NIH HHS/United States
- NS056218/NS/NINDS NIH HHS/United States
- AG038747/AG/NIA NIH HHS/United States
- P51 OD011106/OD/NIH HHS/United States
- R01 AT006526/AT/NCCIH NIH HHS/United States
- RR15459-01/RR/NCRR NIH HHS/United States
- R01 AG038747/AG/NIA NIH HHS/United States
- P01 AG11370/AG/NIA NIH HHS/United States
- RR020141-01/RR/NCRR NIH HHS/United States
- AG031085/AG/NIA NIH HHS/United States
- R01 AG026607/AG/NIA NIH HHS/United States

