The spanin complex is essential for lambda lysis
- PMID: 22904283
- PMCID: PMC3458670
- DOI: 10.1128/JB.01245-12
The spanin complex is essential for lambda lysis
Abstract
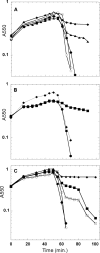
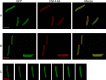
Phage lysis is a ubiquitous biological process, the most frequent cytocidal event in the biosphere. Lysis of Gram-negative hosts has been shown to require holins and endolysins, which attack the cytoplasmic membrane and peptidoglycan, respectively. Recently, a third class of lysis proteins, the spanins, was identified. The first spanins to be characterized were λ Rz and Rz1, an integral cytoplasmic membrane protein and an outer membrane lipoprotein, respectively. Previous work has shown that Rz and Rz1 form complexes that span the entire periplasm. Phase-contrast video microscopy was used to record the morphological changes involved in the lysis of induced λ lysogens carrying prophages with either the λ canonical holin-endolysin system or the phage 21 pinholin-signal anchor release (SAR) endolysin system. In the former, rod morphology persisted until the instant of an explosive polar rupture, immediately emptying the cell of its contents. In contrast, in pinholin-SAR endolysin lysis, the cell began to shorten and thicken uniformly, with the resultant rounded cell finally bursting. In both cases, lysis failed to occur in inductions of isogenic prophages carrying null mutations in the spanin genes. In both systems, instead of an envelope rupture, the induced cells were converted from a rod shape to a spherical form. A functional GFPΦRz chimera was shown to exhibit a punctate distribution when coexpressed with Rz1, despite the absence of endolysin function. A model is proposed in which the spanins carry out the essential step of disrupting the outer membrane, in a manner regulated by the state of the peptidoglycan layer.
Figures


References
-
- Abedon ST. 1989. Selection for bacteriophage latent period length by bacterial density: a theoretical examination. Microbiol. Ecol. 18:79–88 - PubMed
-
- Abramoff MD, Magalhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics Int. 11:36–42
-
- Bull JJ, Pfennig DW, Wang IN. 2004. Genetic details, optimization and phage life histories. Trends Ecol. Evol. 19:76–82 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

