Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells
- PMID: 22904319
- PMCID: PMC3464549
- DOI: 10.1074/jbc.M112.392084
Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells
Abstract
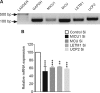
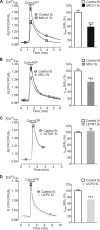
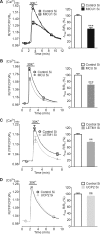
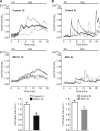
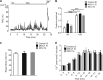
In pancreatic β-cells, uptake of Ca(2+) into mitochondria facilitates metabolism-secretion coupling by activation of various matrix enzymes, thus facilitating ATP generation by oxidative phosphorylation and, in turn, augmenting insulin release. We employed an siRNA-based approach to evaluate the individual contribution of four proteins that were recently described to be engaged in mitochondrial Ca(2+) sequestration in clonal INS-1 832/13 pancreatic β-cells: the mitochondrial Ca(2+) uptake 1 (MICU1), mitochondrial Ca(2+) uniporter (MCU), uncoupling protein 2 (UCP2), and leucine zipper EF-hand-containing transmembrane protein 1 (LETM1). Using a FRET-based genetically encoded Ca(2+) sensor targeted to mitochondria, we show that a transient knockdown of MICU1 or MCU diminished mitochondrial Ca(2+) uptake upon both intracellular Ca(2+) release and Ca(2+) entry via L-type channels. In contrast, knockdown of UCP2 and LETM1 exclusively reduced mitochondrial Ca(2+) uptake in response to either intracellular Ca(2+) release or Ca(2+) entry, respectively. Therefore, we further investigated the role of MICU1 and MCU in metabolism-secretion coupling. Diminution of MICU1 or MCU reduced mitochondrial Ca(2+) uptake in response to d-glucose, whereas d-glucose-triggered cytosolic Ca(2+) oscillations remained unaffected. Moreover, d-glucose-evoked increases in cytosolic ATP and d-glucose-stimulated insulin secretion were diminished in MICU1- or MCU-silenced cells. Our data highlight the crucial role of MICU1 and MCU in mitochondrial Ca(2+) uptake in pancreatic β-cells and their involvement in the positive feedback required for sustained insulin secretion.
Figures
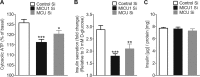
References
-
- Joseph J. W., Jensen M. V., Ilkayeva O., Palmieri F., Alárcon C., Rhodes C. J., Newgard C. B. (2006) The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J. Biol. Chem. 281, 35624–35632 - PubMed
-
- Wiederkehr A., Szanda G., Akhmedov D., Mataki C., Heizmann C. W., Schoonjans K., Pozzan T., Spät A., Wollheim C. B. (2011) Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 13, 601–611 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

