Consolidation and translation regulation
- PMID: 22904372
- PMCID: PMC3418764
- DOI: 10.1101/lm.026849.112
Consolidation and translation regulation
Abstract
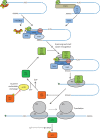
mRNA translation, or protein synthesis, is a major component of the transformation of the genetic code into any cellular activity. This complicated, multistep process is divided into three phases: initiation, elongation, and termination. Initiation is the step at which the ribosome is recruited to the mRNA, and is regarded as the major rate-limiting step in translation, while elongation consists of the elongation of the polypeptide chain; both steps are frequent targets for regulation, which is defined as a change in the rate of translation of an mRNA per unit time. In the normal brain, control of translation is a key mechanism for regulation of memory and synaptic plasticity consolidation, i.e., the off-line processing of acquired information. These regulation processes may differ between different brain structures or neuronal populations. Moreover, dysregulation of translation leads to pathological brain function such as memory impairment. Both normal and abnormal function of the translation machinery is believed to lead to translational up-regulation or down-regulation of a subset of mRNAs. However, the identification of these newly synthesized proteins and determination of the rates of protein synthesis or degradation taking place in different neuronal types and compartments at different time points in the brain demand new proteomic methods and system biology approaches. Here, we discuss in detail the relationship between translation regulation and memory or synaptic plasticity consolidation while focusing on a model of cortical-dependent taste learning task and hippocampal-dependent plasticity. In addition, we describe a novel systems biology perspective to better describe consolidation.
Figures
References
-
- Alcaraz N, Friedrich T, Kotzing T, Krohmer A, Muller J, Pauling J, Baumbach J 2012. Efficient key pathway mining: Combining networks and OMICS data. Integr Biol (Camb) 4: 756–764 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
