Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands
- PMID: 22904561
- PMCID: PMC3477201
- DOI: 10.1124/dmd.112.047530
Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands
Abstract
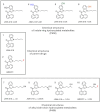
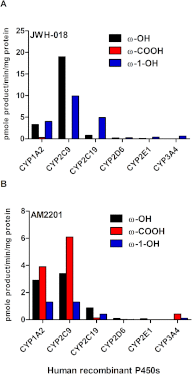
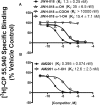
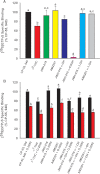
Abuse of synthetic cannabinoids (SCs), such as [1-naphthalenyl-(1-pentyl-1H-indol-3-yl]-methanone (JWH-018) and [1-(5-fluoropentyl)-1H-indol-3-yl]-1-naphthalenyl-methanone (AM2201), is increasing at an alarming rate. Although very little is known about the metabolism and toxicology of these popular designer drugs, mass spectrometric analysis of human urine specimens after JWH-018 and AM2201 exposure identified monohydroxylated and carboxylated derivatives as major metabolites. The present study extends these initial findings by testing the hypothesis that JWH-018 and its fluorinated counterpart AM2201 are subject to cytochrome P450 (P450)-mediated oxidation, forming potent hydroxylated metabolites that retain significant affinity and activity at the cannabinoid 1 (CB(1)) receptor. Kinetic analysis using human liver microsomes and recombinant human protein identified CYP2C9 and CYP1A2 as major P450s involved in the oxidation of the JWH-018 and AM2201. In vitro metabolite formation mirrored human urinary metabolic profiles, and each of the primary enzymes exhibited high affinity (K(m) = 0.81-7.3 μM) and low to high reaction velocities (V(max) = 0.0053-2.7 nmol of product · min(-1) · nmol protein(-1)). The contribution of CYP2C19, 2D6, 2E1, and 3A4 in the hepatic metabolic clearance of these synthetic cannabinoids was minimal (f(m) = <0.2). In vitro studies demonstrated that the primary metabolites produced in humans display high affinity and intrinsic activity at the CB(1) receptor, which was attenuated by the CB(1) receptor antagonist (6aR,10aR)-3-(1-methanesulfonylamino-4-hexyn-6-yl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran (O-2050). Results from the present study provide critical, missing data related to potential toxicological properties of "K2" parent compounds and their human metabolites, including mechanism(s) of action at cannabinoid receptors.
Figures
References
-
- Adams IB, Martin BR. (1996) Cannabis: pharmacology and toxicology in animals and humans. Addiction 91:1585–1614 - PubMed
-
- Banken JA. (2004) Drug abuse trends among youth in the United States. Ann NY Acad Sci 1025:465–471 - PubMed
-
- Benford DM, Caplan JP. (2011) Psychiatric sequelae of Spice, K2, and synthetic cannabinoid receptor agonists. Psychosomatics 52:295. - PubMed
-
- Bland TM, Haining RL, Tracy TS, Callery PS. (2005) CYP2C-catalyzed Δ9-tetrahydrocannabinol metabolism: kinetics, pharmacogenetics and interaction with phenytoin. Biochem Pharmacol 70:1096–1103 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials
Miscellaneous

