Development of a reduction-sensitive diselenide-conjugated oligoethylenimine nanoparticulate system as a gene carrier
- PMID: 22904624
- PMCID: PMC3418076
- DOI: 10.2147/IJN.S32961
Development of a reduction-sensitive diselenide-conjugated oligoethylenimine nanoparticulate system as a gene carrier
Abstract
Background: The reduction-sensitive cationic polymer is a promising nonviral carrier for gene delivery. Until now, disulfide bonds have been the only golden standard for its design. The aim of this research was to develop a novel reduction-responsive cationic polymer as a gene carrier.
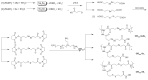
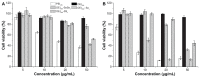
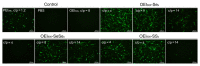
Methods: Polycationic carriers were synthesized by addition of branched oligoethylenimine 800 Da (OEI(800)) via an active ester containing diselenide bonds. Disulfide bonds cross-linked with OEI(800)-SS(x) and monoselenide bonds linked with OEI(800)-Se(x) were synthesized and compared. Their molecular weights and degradation properties were determined using gel permeation chromatography. Changes in particle size, morphology, and DNA binding were investigated by dynamic light scattering, transmission electron microscopy, and electrophoresis assay in a reduction environment. Cytotoxicity and transfection in vitro were evaluated in a murine melanoma cell line (B16F10) and a human cervical epithelial carcinoma cell line (HeLa), while intracellular degradation and dissociation with DNA were studied by confocal laser scanning microscopy with FITC-labeled OEI(800) derivatives and Cy5-labeled DNA.
Results: Diselenide-conjugated OEI(800) (OEI(800)-SeSe(x)) polymer carriers of high molecular weight were successfully synthesized. After compacting with DNA, the OEI(800)-SeSe(x) polymers formed nanoparticles with an average size of 140 nm at an adequate C/P ratio. OEI(800)-SeSe(x) showed reduction-responsive degradation properties similar to those of the OEI(800)-SS(x) via gel permeation chromatography, dynamic light scattering, and transmission electron microscopy. OEI(800)-SeSe(x) showed much lower cytotoxicity than PEI(25k), and significantly higher transfection efficiency than OEI(800) in both B16F10 and HeLa cells. Transfection of luciferase in the OEI(800)-SeSe(x) group was comparable with that of standard PEI(25k) and traditional reduction-sensitive polymer OEI(800)-SS(x) groups. Furthermore, intracellular degradation of OEI(800)-SeSe(x) and dissociation with DNA were also confirmed by confocal laser scanning microscopy.
Conclusion: The OEI(800)-SeSe(x) obtained was able to bind plasmid DNA efficiently to yield nanosized particles and had reduction sensitivity which is as efficient as that for OEI(800)-SS(x). In vitro experiments confirmed its low cytotoxicity and high transfection ability. Diselenide bonds can be used as effective and novel reduction-sensitive linkages for gene delivery.
Keywords: diselenide; gene carriers; oligoethylenimine; reduction-sensitive.
Figures







References
-
- Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2008;109(2):259–302. - PubMed
-
- Sun YX, Xiao W, Cheng SX, Zhang XZ, Zhuo RX. Synthesis of (Dex-HMDI)-g-PEIs as effective and low cytotoxic nonviral gene vectors. J Control Release. 2008;128(2):171–178. - PubMed
-
- Nie Y, Zhang ZR, Duan YR. Combined use of polycationic peptide and biodegradable macromolecular polymer as a novel gene delivery system: a preliminary study. Drug Deliv. 2006;13(6):441–446. - PubMed
-
- Wang XL, Nguyen T, Gillespie D, Jensen R, Lu ZR. A multifunctional and reversibly polymerizable carrier for efficient siRNA delivery. Biomaterials. 2008;29(1):15–22. - PubMed
-
- Pouton CW, Seymour LW. Key issues in non-viral gene delivery. Adv Drug Deliv Rev. 2001;46(1–3):187–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

