Structural and functional difference of pheromone binding proteins in discriminating chemicals in the gypsy moth, Lymantria dispar
- PMID: 22904666
- PMCID: PMC3421229
- DOI: 10.7150/ijbs.4557
Structural and functional difference of pheromone binding proteins in discriminating chemicals in the gypsy moth, Lymantria dispar
Abstract
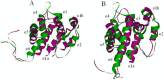
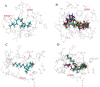
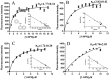
Pheromone-binding proteins (PBPs) of the gypsy moth, Lymantria dispar L., play an important role in olfaction. Here structures of PBPs were first built by Homology Modeling, and each model of PBPs had seven α-helices and a large hydrophobic cavity including 25 residues for PBP1 and 30 residues for PBP2. Three potential semiochemicals were first screened by CDOCKER program based on the PBP models and chemical database. These chemicals were Palmitic acid n-butyl ester (Pal), Bis(3,4-epoxycyclohexylmethyl) adipate (Bis), L-trans-epoxysuccinyl-isoleucyl-proline methyl ester propylamide (CA-074). The analysis of chemicals docking the proteins showed one hydrogen bond was established between the residues Lys94 and (+)-Disparlure ((+)-D), and л-л interactions were present between Phe36 of PBP1 and (+)-D. The Lys94 of PBP1 formed two and three hydrogen bonds with Bis and CA-074, respectively. There was no residue of PBP2 interacting with these four chemicals except Bis forming one hydrogen bond with Lys121. After simulating the conformational changes of LdisPBPs at pH7.3 and 5.5 by constant pH molecular dynamics simulation in implicit solvent, the N-terminal sequences of PBPs was unfolded, only having five α-helices, and PBP2 had larger binding pocket at 7.3 than PBP1. To investigate the changes of α-helices at different pH, far-UV and near-UV circular dichroism showed PBPs consist of α-helices, and the tertiary structures of PBP1 and PBP2 were influenced at pH7.3 and 5.5. The fluorescence binding assay indicated that PBP1 and PBP2 have similarly binding affinity to (+)-D at pH 5.5 and 7.3, respectively. At pH 5.5, the dissociation constant of the complex between PBP1 and 2-decyl-1-oxaspiro [2.2] pentane (OXP1) was 0.68 ± 0.01 μM, for (+)-D was 5.32 ± 0.11 μM, while PBP2 with OXP1 and (+)-D were 1.88 ± 0.02 μM and 5.54 ± 0.04 μM, respectively. Three chemicals screened had higher affinity to PBP1 than (+)-D except Pal at pH5.5, and had lower affinity than (+)-D at pH7.3. To PBP2, these chemicals had lower affinity than the sex pheromone except Bis at pH 5.5 and pH 7.3. Only PBP1 had higher affinity with Sal than the sex pheromone at pH 5.5. Therefore, the structures of PBP1 and PBP2 had different changes at pH5.5 and 7.3, showing different affinity to chemicals. This study helps understanding the role of PBPs as well as in developing more efficient chemicals for pest control.
Keywords: Lymantria dispar; bioinformatics; discrimination; pheromone-binding protein; semiochemicals.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Kaissling KE. Peripheral mechanism of pheromone reception in moths. Chem Senses. 1996;21:257–268. - PubMed
-
- Vogt RG. The molecular basis of pheromone reception: its influence on behavior. In: Prestwich GD, Blomquist GJ, editors. Pheromone Biochemistry. New York: Academic Press; 1987. pp. 385–431.
-
- Xu P, Atkinson R, Jones DNM. et al. Drosophila OBP LUSH is required for activity of pheromone-sensitive nerurons. Neuron. 2005;45:193–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

