Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes
- PMID: 22905105
- PMCID: PMC3414482
- DOI: 10.1371/journal.pone.0041756
Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes
Abstract
Background: No targeted immunotherapies reverse type 1 diabetes in humans. However, in a rodent model of type 1 diabetes, Bacillus Calmette-Guerin (BCG) reverses disease by restoring insulin secretion. Specifically, it stimulates innate immunity by inducing the host to produce tumor necrosis factor (TNF), which, in turn, kills disease-causing autoimmune cells and restores pancreatic beta-cell function through regeneration.
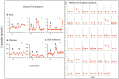
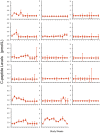
Methodology/principal findings: Translating these findings to humans, we administered BCG, a generic vaccine, in a proof-of-principle, double-blind, placebo-controlled trial of adults with long-term type 1 diabetes (mean: 15.3 years) at one clinical center in North America. Six subjects were randomly assigned to BCG or placebo and compared to self, healthy paired controls (n = 6) or reference subjects with (n = 57) or without (n = 16) type 1 diabetes, depending upon the outcome measure. We monitored weekly blood samples for 20 weeks for insulin-autoreactive T cells, regulatory T cells (Tregs), glutamic acid decarboxylase (GAD) and other autoantibodies, and C-peptide, a marker of insulin secretion. BCG-treated patients and one placebo-treated patient who, after enrollment, unexpectedly developed acute Epstein-Barr virus infection, a known TNF inducer, exclusively showed increases in dead insulin-autoreactive T cells and induction of Tregs. C-peptide levels (pmol/L) significantly rose transiently in two BCG-treated subjects (means: 3.49 pmol/L [95% CI 2.95-3.8], 2.57 [95% CI 1.65-3.49]) and the EBV-infected subject (3.16 [95% CI 2.54-3.69]) vs.1.65 [95% CI 1.55-3.2] in reference diabetic subjects. BCG-treated subjects each had more than 50% of their C-peptide values above the 95(th) percentile of the reference subjects. The EBV-infected subject had 18% of C-peptide values above this level.
Conclusions/significance: We conclude that BCG treatment or EBV infection transiently modified the autoimmunity that underlies type 1 diabetes by stimulating the host innate immune response. This suggests that BCG or other stimulators of host innate immunity may have value in the treatment of long-term diabetes.
Trial registration: ClinicalTrials.gov NCT00607230.
Conflict of interest statement
Figures
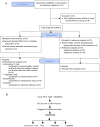


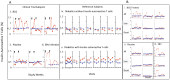

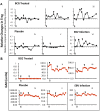
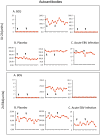
Comment in
-
Targeting innate immunity to treat long-term type 1 diabetes.Regen Med. 2012 Nov;7(6):753-4. Regen Med. 2012. PMID: 23304723 No abstract available.
Similar articles
-
TNF, TNF inducers, and TNFR2 agonists: A new path to type 1 diabetes treatment.Diabetes Metab Res Rev. 2018 Jan;34(1). doi: 10.1002/dmrr.2941. Epub 2017 Sep 29. Diabetes Metab Res Rev. 2018. PMID: 28843039 Review.
-
Effect of Bacillus Calmette-Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study.Diabetes Care. 1999 Oct;22(10):1703-7. doi: 10.2337/diacare.22.10.1703. Diabetes Care. 1999. PMID: 10526739 Clinical Trial.
-
Avoiding COVID-19 complications with diabetic patients could be achieved by multi-dose Bacillus Calmette-Guérin vaccine: a case study of beta cells regeneration.Pharmazie. 2020 Aug 1;75(8):375-380. doi: 10.1691/ph.2020.0494. Pharmazie. 2020. PMID: 32758336
-
Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial.Lancet. 2011 Jul 23;378(9788):319-27. doi: 10.1016/S0140-6736(11)60895-7. Epub 2011 Jun 27. Lancet. 2011. PMID: 21714999 Free PMC article. Clinical Trial.
-
Significance of Bacillus Calmette-Guerin (BCG) vaccine intervention for patients with Type 1 Diabetes (T1D): A systematic review and meta-analysis.Diabetes Metab Syndr. 2024 Aug;18(8):103102. doi: 10.1016/j.dsx.2024.103102. Epub 2024 Aug 12. Diabetes Metab Syndr. 2024. PMID: 39173532
Cited by
-
BCG vaccinations drive epigenetic changes to the human T cell receptor: Restored expression in type 1 diabetes.Sci Adv. 2022 Nov 18;8(46):eabq7240. doi: 10.1126/sciadv.abq7240. Epub 2022 Nov 16. Sci Adv. 2022. PMID: 36383663 Free PMC article.
-
BCG Vaccination: A potential tool against COVID-19 and COVID-19-like Black Swan incidents.Int Immunopharmacol. 2022 Jul;108:108870. doi: 10.1016/j.intimp.2022.108870. Epub 2022 May 17. Int Immunopharmacol. 2022. PMID: 35597119 Free PMC article. Review.
-
Vaccine for Diabetes-Where Do We Stand?Int J Mol Sci. 2022 Aug 22;23(16):9470. doi: 10.3390/ijms23169470. Int J Mol Sci. 2022. PMID: 36012735 Free PMC article. Review.
-
TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine.Front Immunol. 2013 Dec 23;4:478. doi: 10.3389/fimmu.2013.00478. Front Immunol. 2013. PMID: 24391650 Free PMC article.
-
Effects of Bacillus Calmette-Guérin on immunometabolism, microbiome and liver diseases⋆.Liver Res. 2023 Jun;7(2):116-123. doi: 10.1016/j.livres.2023.05.001. Epub 2023 May 19. Liver Res. 2023. PMID: 38223885 Free PMC article.
References
-
- Grewal IS, Grewal KD, Wong FS, Picarella DE, Janeway CA, et al. (1996) Local expression of transgene encoded TNF alpha in islets prevents autoimmune diabetes in non-obese diabetic (NOD) mice by preventing the development of autoreactive islet specific T cells. J Exp Med 184: 1963–1974. - PMC - PubMed
-
- Qin HY, Chaturvedi P, Singh B (2004) In vivo apoptosis of diabetogenic T cells in NOD mice by IFN-γ/TNF-α. Int Immunol 16: 1723–1732. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

