Salmon aquaculture and antimicrobial resistance in the marine environment
- PMID: 22905164
- PMCID: PMC3414459
- DOI: 10.1371/journal.pone.0042724
Salmon aquaculture and antimicrobial resistance in the marine environment
Abstract
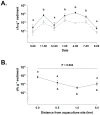
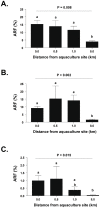
Antimicrobials used in salmon aquaculture pass into the marine environment. This could have negative impacts on marine environmental biodiversity, and on terrestrial animal and human health as a result of selection for bacteria containing antimicrobial resistance genes. We therefore measured the numbers of culturable bacteria and antimicrobial-resistant bacteria in marine sediments in the Calbuco Archipelago, Chile, over 12-month period at a salmon aquaculture site approximately 20 m from a salmon farm and at a control site 8 km distant without observable aquaculture activities. Three antimicrobials extensively used in Chilean salmon aquaculture (oxytetracycline, oxolinic acid, and florfenicol) were studied. Although none of these antimicrobials was detected in sediments from either site, traces of flumequine, a fluoroquinolone antimicrobial also widely used in Chile, were present in sediments from both sites during this period. There were significant increases in bacterial numbers and antimicrobial-resistant fractions to oxytetracycline, oxolinic acid, and florfenicol in sediments from the aquaculture site compared to those from the control site. Interestingly, there were similar numbers of presumably plasmid-mediated resistance genes for oxytetracycline, oxolinic acid and florfenicol in unselected marine bacteria isolated from both aquaculture and control sites. These preliminary findings in one location may suggest that the current use of large amounts of antimicrobials in Chilean aquaculture has the potential to select for antimicrobial-resistant bacteria in marine sediments.
Conflict of interest statement
Figures



References
-
- Costa-Pierce BA (2010) Sustainable ecological aquaculture systems: the need for a new social contract for aquaculture development. Mar Technol Soc J 44: 88–112.
-
- Tett P (2008) Fish farm wastes in the ecosystem. In: Holmer M, Black K, Duarte CM, Marbà N, Karakassis I, editors. Aquaculture in the Ecosystem. Berlin Heidelberg, German: Springer-Verlag. 1–46.
-
- Moore PR, Evenson A, Luckey TD, McCoy E, Elvehjemande CA, et al. (1946) Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J Biol Chem 165: 437–441. - PubMed
-
- Grumbles LC, Delaplane JP, Higgins TC (1948) Sulfaquinoxaline in the control of Eimeria tenella and Eimeria necatrix in chickens on a commercial broiler farm. Science 107: 196. - PubMed
-
- Jukes TH, Stokstad ELR, Taylor RR, Cunha TJ, Edwards HM, et al. (1950) Growth promoting effect of aureomycin on pigs. Arch Biochem 26: 324–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

