Glycerol-3-phosphate acyltransferase-2 is expressed in spermatic germ cells and incorporates arachidonic acid into triacylglycerols
- PMID: 22905194
- PMCID: PMC3414494
- DOI: 10.1371/journal.pone.0042986
Glycerol-3-phosphate acyltransferase-2 is expressed in spermatic germ cells and incorporates arachidonic acid into triacylglycerols
Abstract
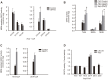
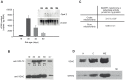
Background: De novo glycerolipid synthesis begins with the acylation of glycerol-3 phosphate catalyzed by glycerol-3-phosphate acyltransferase (GPAT). In mammals, at least four GPAT isoforms have been described, differing in their cell and tissue locations and sensitivity to sulfhydryl reagents. In this work we show that mitochondrial GPAT2 overexpression in CHO-K1 cells increased TAG content and both GPAT and AGPAT activities 2-fold with arachidonoyl-CoA as a substrate, indicating specificity for this fatty acid.
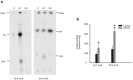
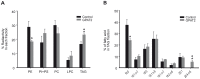
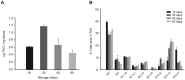
Methods and results: Incubation of GPAT2-transfected CHO-K1 cells with [1-(14)C]arachidonate for 3 h increased incorporation of [(14)C]arachidonate into TAG by 40%. Consistently, arachidonic acid was present in the TAG fraction of cells that overexpressed GPAT2, but not in control cells, corroborating GPAT2's role in synthesizing TAG that is rich in arachidonic acid. In rat and mouse testis, Gpat2 mRNA was expressed only in primary spermatocytes; the protein was also detected in late stages of spermatogenesis. During rat sexual maturation, both the testicular TAG content and the arachidonic acid content in the TAG fraction peaked at 30 d, matching the highest expression of Gpat2 mRNA and protein.
Conclusions: These results strongly suggest that GPAT2 expression is linked to arachidonoyl-CoA incorporation into TAG in spermatogenic germ cells.
Conflict of interest statement
Figures



References
-
- Lewin TM, Schwerbrock NM, Lee DP, Coleman RA (2004) Identification of a new glycerol-3-phosphate acyltransferase isoenzyme, mtGPAT2, in mitochondria. J Biol Chem 279: 13488–13495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

