Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation
- PMID: 22905233
- PMCID: PMC3419202
- DOI: 10.1371/journal.pone.0043197
Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation
Abstract
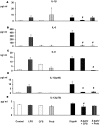
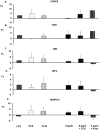
Probiotic bacteria have been shown to modulate immune responses and could have therapeutic effects in allergic and inflammatory disorders. However, little is known about the signalling pathways that are engaged by probiotics. Dendritic cells (DCs) are antigen-presenting cells that are involved in immunity and tolerance. Monocyte-derived dendritic cells (MoDCs) and murine DCs are different from human gut DCs; therefore, in this study, we used human DCs generated from CD34+ progenitor cells (hematopoietic stem cells) harvested from umbilical cord blood; those DCs exhibited surface antigens of dendritic Langerhans cells, similar to the lamina propria DCs in the gut. We report that both a novel probiotic strain isolated from faeces of exclusively breast-fed newborn infants, Lactobacillus paracasei CNCM I-4034, and its cell-free culture supernatant (CFS) decreased pro-inflammatory cytokines and chemokines in human intestinal DCs challenged with Salmonella. Interestingly, the supernatant was as effective as the bacteria in reducing pro-inflammatory cytokine expression. In contrast, the bacterium was a potent inducer of TGF-β2 secretion, whereas the supernatant increased the secretion of TGF-β1 in response to Salmonella. We also showed that both the bacteria and its supernatant enhanced innate immunity through the activation of Toll-like receptor (TLR) signalling. These treatments strongly induced the transcription of the TLR9 gene. In addition, upregulation of the CASP8 and TOLLIP genes was observed. This work demonstrates that L. paracasei CNCM I-4034 enhanced innate immune responses, as evidenced by the activation of TLR signalling and the downregulation of a broad array of pro-inflammatory cytokines. The use of supernatants like the one described in this paper could be an effective and safe alternative to using live bacteria in functional foods.
Conflict of interest statement
Figures





References
-
- FAO/WHO (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria.
-
- Collado MC, Isolauri E, Salminen S, Sanz Y (2009) The impact of probiotic on gut health. Curr. Drug. Metab. 10: 68–78. - PubMed
-
- Lebeer S, Vanderleyden J, De Keersmaecker SC (2010) Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8: 171–84. - PubMed
-
- Bongaerts GP, Severijnen RS (2005) Preventive and curative effects of probiotics in atopic patients. Med. Hypotheses. 64: 1089–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

