GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC
- PMID: 22905242
- PMCID: PMC3419200
- DOI: 10.1371/journal.pone.0043255
GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC
Abstract
Background: Mesenchymal stromal cells (MSC) have gained importance in tissue repair, tissue engineering and in immunosupressive therapy during the last years. Due to the limited availability of MSC in the bone marrow, ex vivo amplification prior to clinical application is requisite to obtain therapeutic applicable cell doses. Translation of preclinical into clinical-grade large-scale MSC expansion necessitates precise definition and standardization of all procedural parameters including cell seeding density, culture medium and cultivation devices. While xenogeneic additives such as fetal calf serum are still widely used for cell culture, its use in the clinical context is associated with many risks, such as prion and viral transmission or adverse immunological reactions against xenogeneic components.
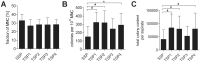
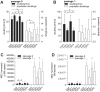
Methods and findings: We established animal-free expansion protocols using platelet lysate as medium supplement and thereby could confirm its safety and feasibility for large-scale MSC isolation and expansion. Five different GMP-compliant standardized protocols designed for the safe, reliable, efficient and economical isolation and expansion of MSC was performed and MSC obtained were analyzed for differentiation capacity by qPCR and histochemistry. Expression of standard MSC markers as defined by the International Society for Cellular Therapy as well as expression of additional MSC markers and of various chemokine and cytokine receptors was analysed by flow cytometry. Changes of metabolic markers and cytokines in the medium were addressed using the LUMINEX platform.
Conclusions: The five different systems for isolation and expansion of MSC described in this study are all suitable to produce at least 100 millions of MSC, which is commonly regarded as a single clinical dose. Final products are equal according to the minimal criteria for MSC defined by the ISCT. We showed that chemokine and integrin receptors analyzed had the same expression pattern, suggesting that MSC from either of the systems show equal characteristics of homing and adhesion.
Conflict of interest statement
Figures





References
-
- Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317. - PubMed
-
- Caplan AI (1994) The mesengenic process. ClinPlastSurg 21: 429–435. - PubMed
-
- Subbanna PK (2007) Mesenchymal stem cells for treating GVHD: in-vivo fate and optimal dose. MedHypotheses 69: 469–470. - PubMed
-
- EuropeanCommission (2007) REGULATION (EC) No 1394/2007 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Official Journal of the European Union 50: 121–137.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

