Early patterning in a chondrichthyan model, the small spotted dogfish: towards the gnathostome ancestral state
- PMID: 22905913
- PMCID: PMC3552415
- DOI: 10.1111/j.1469-7580.2012.01552.x
Early patterning in a chondrichthyan model, the small spotted dogfish: towards the gnathostome ancestral state
Abstract
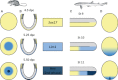
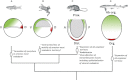
In the past few years, the small spotted dogfish has become the primary model for analyses of early development in chondrichthyans. Its phylogenetic position makes it an ideal outgroup to reconstruct the ancestral gnathostome state by comparisons with established vertebrate model organisms. It is also a suitable model to address the molecular bases of lineage-specific diversifications such as the rise of extraembryonic tissues, as it is endowed with a distinct extraembryonic yolk sac and yolk duct ensuring exchanges between the embryo and a large undivided vitelline mass. Experimental or functional approaches such as cell marking or in ovo pharmacological treatments are emerging in this species, but recent analyses of early development in this species have primarily concentrated on molecular descriptions. These data show the dogfish embryo exhibits early polarities reflecting the dorso-ventral axis of amphibians and teleosts at early blastula stages and an atypical anamniote molecular pattern during gastrulation, independently of the presence of extraembryonic tissues. They also highlight unexpected relationships with amniotes, with a strikingly similar Nodal-dependent regional pattern in the extraembryonic endoderm. In this species, extraembryonic cell fates seem to be determined by differential cell behaviors, which lead to cell allocation in extraembryonic and embryonic tissues, rather than by cell regional identity. We suggest that this may exemplify an early evolutionary step in the rise of extraembryonic tissues, possibly related to quantitative differences in the signaling activities, which shape the early embryo. These results highlight the conservation across gnathostomes of a highly constrained core genetic program controlling early patterning. This conservation may be obscured in some lineages by taxa-specific diversifications such as specializations of extraembryonic nutritive tissues.
© 2012 The Authors. Journal of Anatomy © 2012 Anatomical Society.
Figures




Similar articles
-
Evolution of axis specification mechanisms in jawed vertebrates: insights from a chondrichthyan.PLoS One. 2007 Apr 18;2(4):e374. doi: 10.1371/journal.pone.0000374. PLoS One. 2007. PMID: 17440610 Free PMC article.
-
Towards a synthetic view of axis specification mechanisms in vertebrates: insights from the dogfish.C R Biol. 2009 Feb-Mar;332(2-3):210-8. doi: 10.1016/j.crvi.2008.07.008. Epub 2008 Nov 28. C R Biol. 2009. PMID: 19281952 Review.
-
The evolution of gnathostome development: Insight from chondrichthyan embryology.Genesis. 2009 Dec;47(12):825-41. doi: 10.1002/dvg.20567. Genesis. 2009. PMID: 19882670 Review.
-
Characterization of Brachyury genes in the dogfish S. canicula and the lamprey L. fluviatilis. Insights into gastrulation in a chondrichthyan.Dev Biol. 2003 Nov 15;263(2):296-307. doi: 10.1016/j.ydbio.2003.07.009. Dev Biol. 2003. PMID: 14597203
-
The Dogfish Scyliorhinus canicula: A Reference in Jawed Vertebrates.CSH Protoc. 2008 Dec 1;2008:pdb.emo111. doi: 10.1101/pdb.emo111. CSH Protoc. 2008. PMID: 21356737
Cited by
-
Enigmatic Nodal and Lefty gene repertoire discrepancy: Latent evolutionary history revealed by vertebrate-wide phylogeny.Dev Dyn. 2025 Aug;254(8):887-901. doi: 10.1002/dvdy.710. Epub 2024 Apr 22. Dev Dyn. 2025. PMID: 38647085 Free PMC article. Review.
-
Mechanisms of endoderm formation in a cartilaginous fish reveal ancestral and homoplastic traits in jawed vertebrates.Biol Open. 2014 Oct 31;3(11):1098-107. doi: 10.1242/bio.20148037. Biol Open. 2014. PMID: 25361580 Free PMC article.
References
-
- Albazerchi A, Stern CD. A role for the hypoblast (AVE) in the initiation of neural induction, independent of its ability to position the primitive streak. Dev Biol. 2007;301:489–503. - PubMed
-
- Aoki TO, David NB, Minchiotti G, et al. Molecular integration of casanova in the Nodal signalling pathway controlling endoderm formation. Development. 2002;129:275–286. - PubMed
-
- Arendt D, Nubler-Jung K. Rearranging gastrulation in the name of yolk: evolution of gastrulation in yolk-rich amniote eggs. Mech Dev. 1999;81:3–22. - PubMed
-
- Bachiller D, Klingensmith J, Kemp C, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. - PubMed
-
- Ballard W, Mellinger J, Lechenaud H. A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish (Shondrichtyes: Scyliorhinidae) J Exp Zool. 1993;267:318–336.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

