Molecular cloning and functional characterization of an ATP-binding cassette transporter OtrC from Streptomyces rimosus
- PMID: 22906146
- PMCID: PMC3533511
- DOI: 10.1186/1472-6750-12-52
Molecular cloning and functional characterization of an ATP-binding cassette transporter OtrC from Streptomyces rimosus
Abstract
Background: The otrC gene of Streptomyces rimosus was previously annotated as an oxytetracycline (OTC) resistance protein. However, the amino acid sequence analysis of OtrC shows that it is a putative ATP-binding cassette (ABC) transporter with multidrug resistance function. To our knowledge, none of the ABC transporters in S. rimosus have yet been characterized. In this study, we aimed to characterize the multidrug exporter function of OtrC and evaluate its relevancy to OTC production.
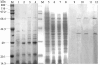
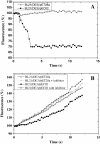
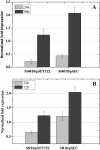
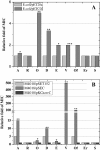
Results: In order to investigate OtrC's function, otrC is cloned and expressed in E. coli The exporter function of OtrC was identified by ATPase activity determination and ethidium bromide efflux assays. Also, the susceptibilities of OtrC-overexpressing cells to several structurally unrelated drugs were compared with those of OtrC-non-expressing cells by minimal inhibitory concentration (MIC) assays, indicating that OtrC functions as a drug exporter with a broad range of drug specificities. The OTC production was enhanced by 1.6-fold in M4018 (P = 0.000877) and 1.4-fold in SR16 (P = 0.00973) duplication mutants, while it decreased to 80% in disruption mutants (P = 0.0182 and 0.0124 in M4018 and SR16, respectively).
Conclusions: The results suggest that OtrC is an ABC transporter with multidrug resistance function, and plays an important role in self-protection by drug efflux mechanisms. This is the first report of such a protein in S. rimosus, and otrC could be a valuable target for genetic manipulation to improve the production of industrial antibiotics.
Figures




Similar articles
-
Identification of a cluster-situated activator of oxytetracycline biosynthesis and manipulation of its expression for improved oxytetracycline production in Streptomyces rimosus.Microb Cell Fact. 2015 Apr 2;14:46. doi: 10.1186/s12934-015-0231-7. Microb Cell Fact. 2015. PMID: 25886456 Free PMC article.
-
Improvement of oxytetracycline production mediated via cooperation of resistance genes in Streptomyces rimosus.Sci China Life Sci. 2017 Sep;60(9):992-999. doi: 10.1007/s11427-017-9121-4. Epub 2017 Jul 26. Sci China Life Sci. 2017. PMID: 28755296
-
Role of Aromatic and Negatively Charged Residues of DrrB in Multisubstrate Specificity Conferred by the DrrAB System of Streptomyces peucetius.Biochemistry. 2017 Apr 4;56(13):1921-1931. doi: 10.1021/acs.biochem.6b01155. Epub 2017 Mar 20. Biochemistry. 2017. PMID: 28272881
-
Structure and mechanism of ABC transporters.Curr Opin Struct Biol. 2002 Dec;12(6):754-60. doi: 10.1016/s0959-440x(02)00399-8. Curr Opin Struct Biol. 2002. PMID: 12504680 Review.
-
Structure and function of multidrug transporters.Adv Exp Med Biol. 1998;456:145-58. doi: 10.1007/978-1-4615-4897-3_8. Adv Exp Med Biol. 1998. PMID: 10549367 Review. No abstract available.
Cited by
-
Identification of a cluster-situated activator of oxytetracycline biosynthesis and manipulation of its expression for improved oxytetracycline production in Streptomyces rimosus.Microb Cell Fact. 2015 Apr 2;14:46. doi: 10.1186/s12934-015-0231-7. Microb Cell Fact. 2015. PMID: 25886456 Free PMC article.
-
Characterization of a Bi-directional Promoter OtrRp Involved in Oxytetracycline Biosynthesis.Curr Microbiol. 2019 Nov;76(11):1264-1269. doi: 10.1007/s00284-019-01753-1. Epub 2019 Aug 13. Curr Microbiol. 2019. PMID: 31410507
-
A Putative Efflux Transporter of the ABC Family, YbhFSR, in Escherichia coli Functions in Tetracycline Efflux and Na+(Li+)/H+ Transport.Front Microbiol. 2020 Apr 23;11:556. doi: 10.3389/fmicb.2020.00556. eCollection 2020. Front Microbiol. 2020. PMID: 32390957 Free PMC article.
-
Metagenomic immunoglobulin sequencing reveals IgA coating of microbial strains in the healthy human gut.Nat Microbiol. 2025 Jan;10(1):112-125. doi: 10.1038/s41564-024-01887-4. Epub 2025 Jan 2. Nat Microbiol. 2025. PMID: 39747692
-
Biosynthesis of Oxytetracycline by Streptomyces rimosus: Past, Present and Future Directions in the Development of Tetracycline Antibiotics.Food Technol Biotechnol. 2017 Mar;55(1):3-13. doi: 10.17113/ftb.55.01.17.4617. Food Technol Biotechnol. 2017. PMID: 28559729 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

