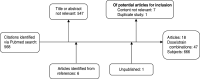
The Shigella human challenge model
- PMID: 22906296
- PMCID: PMC9152033
- DOI: 10.1017/S0950268812001677
The Shigella human challenge model
Abstract
Shigella is an important bacterial cause of infectious diarrhoea globally. The Shigella human challenge model has been used since 1946 for a variety of objectives including understanding disease pathogenesis, human immune responses and allowing for an early assessment of vaccine efficacy. A systematic review of the literature regarding experimental shigellosis in human subjects was conducted. Summative estimates were calculated by strain and dose. While a total of 19 studies evaluating nine strains at doses ranging from 10 to 1 × 1010 colony-forming units were identified, most studies utilized the S. sonnei strain 53G and the S. flexneri strain 2457T. Inoculum solution and pre-inoculation buffering has varied over time although diarrhoea attack rates do not appear to increase above 75-80%, and dysentery rates remain fairly constant, highlighting the need for additional dose-ranging studies. Expansion of the model to include additional strains from different serotypes will elucidate serotype and strain-specific outcome variability.
Figures
References
-
- Riddle MS, et al. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. American Journal of Tropical Medicine and Hygiene 2006; 74: 891–900. - PubMed
-
- Shaughnessy HJ, et al. Experimental human bacillary dysentery; polyvalent dysentery vaccine in its prevention. Journal of the American Medical Association 1946; 132: 362–368. - PubMed
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986; 7: 177–188. - PubMed
-
- Van de Verg LL, et al. Cross-reactivity of Shigella flexneri serotype 2a O antigen antibodies following immunization or infection. Vaccine 1996; 14: 1062–1068. - PubMed